Antioxidant, anti-inflammatory, anti-apoptotic, and skin regenerative properties of an Aloe vera-based extract of Nerium oleander leaves (nae-8(®))
- PMID: 26005354
- PMCID: PMC4427598
- DOI: 10.2147/CCID.S79871
Antioxidant, anti-inflammatory, anti-apoptotic, and skin regenerative properties of an Aloe vera-based extract of Nerium oleander leaves (nae-8(®))
Abstract
Objective: The goal for this study was to evaluate the effects of an Aloe vera-based Nerium oleander extract (NAE-8(®)), compared to an extract of A. vera gel alone (ALOE), and to an aqueous extract of N. oleander (AQ-NOE) in bioassays pertaining to dermatologic potential with respect to antioxidant protection, anti-inflammatory effects, and cytokine profiles in vitro.
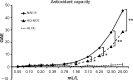
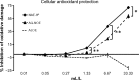
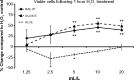
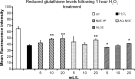
Methods: Cellular antioxidant protection was evaluated in three separate bioassays: The cellular antioxidant protection of erythrocytes (CAP-e) assay, protection of cellular viability and prevention of apoptosis, and protection of intracellular reduced glutathione levels, where the last two assays were performed using human primary dermal fibroblasts. Reduction of intracellular formation of reactive oxygen species (ROS) was tested using polymorphonuclear cells in the absence and presence of oxidative stress. Changes to cytokine and chemokine profiles when whole blood cells and human primary dermal fibroblasts were exposed to test products were determined using a 40-plex Luminex array as a method for exploring the potential cross-talk between circulating and skin-resident cells.
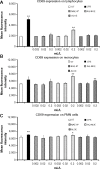
Results: The NAE-8(®) provided significantly better antioxidant protection in the CAP-e bioassay than AQ-NOE. NAE-8(®) and AQ-NOE both protected cellular viability and intracellular reduced glutathione, and reduced the ROS formation significantly when compared to control cells, both under inflamed and neutral culture conditions. ALOE showed minimal effect in these bioassays. In contrast to the NAE-8(®), the AQ-NOE showed induction of inflammation in the whole blood cultures, as evidenced by the high induction of CD69 expression and secretion of a number of inflammatory cytokines. The treatment of dermal fibroblasts with NAE-8(®) resulted in selective secretion of cytokines involved in collagen and hyaluronan production as well as re-epithelialization during wound healing.
Conclusion: NAE-8(®), a novel component of a commercial cosmetic product, showed beneficial antioxidant protection in several cellular models, without the induction of leukocyte activation and secretion of inflammatory cytokines. The biological efficacy of NAE-8(®) was unique from both ALOE and AQ-NOE.
Keywords: CAP-e bioassay; ROS formation; dermal fibroblasts; oxidative damage; safety.
Figures



Similar articles
-
Evaluation of the wound healing potential of Aloe vera-based extract of Nerium oleander.North Clin Istanb. 2017 Oct 19;4(3):205-212. doi: 10.14744/nci.2017.94914. eCollection 2017. North Clin Istanb. 2017. PMID: 29270567 Free PMC article.
-
Water-soluble egg membrane enhances the immunoactivating properties of an Aloe vera-based extract of Nerium oleander leaves.Clin Cosmet Investig Dermatol. 2016 Nov 3;9:393-403. doi: 10.2147/CCID.S114471. eCollection 2016. Clin Cosmet Investig Dermatol. 2016. PMID: 27843333 Free PMC article.
-
In-vitro assessment and pharmacodynamics of nimesulide incorporated Aloe vera transemulgel.Curr Drug Discov Technol. 2014 Jun;11(2):162-7. doi: 10.2174/1570163810666131202233721. Curr Drug Discov Technol. 2014. PMID: 24295369
-
Final report on the safety assessment of AloeAndongensis Extract, Aloe Andongensis Leaf Juice,aloe Arborescens Leaf Extract, Aloe Arborescens Leaf Juice, Aloe Arborescens Leaf Protoplasts, Aloe Barbadensis Flower Extract, Aloe Barbadensis Leaf, Aloe Barbadensis Leaf Extract, Aloe Barbadensis Leaf Juice,aloe Barbadensis Leaf Polysaccharides, Aloe Barbadensis Leaf Water, Aloe Ferox Leaf Extract, Aloe Ferox Leaf Juice, and Aloe Ferox Leaf Juice Extract.Int J Toxicol. 2007;26 Suppl 2:1-50. doi: 10.1080/10915810701351186. Int J Toxicol. 2007. PMID: 17613130 Review.
-
[Anti-inflammatory constituents, aloesin and aloemannan in Aloe species and effects of tanshinon VI in Salvia miltiorrhiza on heart].Yakugaku Zasshi. 2003 Jul;123(7):517-32. doi: 10.1248/yakushi.123.517. Yakugaku Zasshi. 2003. PMID: 12875235 Review. Japanese.
Cited by
-
The mycelium of the Trametes versicolor (Turkey tail) mushroom and its fermented substrate each show potent and complementary immune activating properties in vitro.BMC Complement Altern Med. 2019 Dec 2;19(1):342. doi: 10.1186/s12906-019-2681-7. BMC Complement Altern Med. 2019. PMID: 31791317 Free PMC article.
-
Regulatory effect of chrysin on expression of lenticular calcium transporters, calpains, and apoptotic-cascade components in selenite-induced cataract.Mol Vis. 2016 Apr 30;22:401-23. eCollection 2016. Mol Vis. 2016. PMID: 27168717 Free PMC article.
-
Evaluation of the wound healing potential of Aloe vera-based extract of Nerium oleander.North Clin Istanb. 2017 Oct 19;4(3):205-212. doi: 10.14744/nci.2017.94914. eCollection 2017. North Clin Istanb. 2017. PMID: 29270567 Free PMC article.
-
Periplaneta americana Extracts Promote Skin Wound Healing via Nuclear Factor Kappa B Canonical Pathway and Extracellular Signal-Regulated Kinase Signaling.Evid Based Complement Alternat Med. 2017;2017:5821706. doi: 10.1155/2017/5821706. Epub 2017 May 23. Evid Based Complement Alternat Med. 2017. PMID: 28620419 Free PMC article.
-
Antiviral activity of oleandrin and a defined extract of Nerium oleander against SARS-CoV-2.Biomed Pharmacother. 2021 Jun;138:111457. doi: 10.1016/j.biopha.2021.111457. Epub 2021 Mar 3. Biomed Pharmacother. 2021. PMID: 33721754 Free PMC article.
References
-
- Fowler JF, Jr, Woolery-Lloyd H, Waldorf H, Saini R. Innovations in natural ingredients and their use in skin care. J Drugs Dermatol. 2010;9(Suppl 6):S72–S81. - PubMed
-
- Moniruzzaman M, Roykeya B, Ahmed S, Bhowmik A, Khalil MI, Gan SH. In vitro antioxidant effects of Aloe barbadensis Miller extracts and the potential role of these extracts as antidiabetic and antilipidemic agents on streptozotocin-induced type 2 diabetic model rats. Molecules. 2012;17(11):12851–12867. - PMC - PubMed
-
- Hossain MS, Mamun-Or-Rashid ANM, Tofique NM, Sen MK. A review on ethnopharmacological potential of Aloe vera L. J Intercult Ethnopharmacol. 2013;2(2):113–120.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

