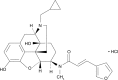
Nalfurafine hydrochloride to treat pruritus: a review
- PMID: 26005355
- PMCID: PMC4433050
- DOI: 10.2147/CCID.S55942
Nalfurafine hydrochloride to treat pruritus: a review
Abstract
Uremic pruritus has a great negative influence on quality of life in hemodialysis (HD) patients and, importantly, negatively affects mortality risk. Recently, nalfurafine hydrochloride, an opioid κ-selective agonist, has been officially approved for resistant pruritus in HD patients on the basis of a well-evidenced clinical trial in Japan. From clinical observation, it has been suggested that the upper neuron system plays a role in its pathogenesis. According to previous experimental results, using mice injected with opioids, dynorphin suppresses itch through binding κ-opioid receptors, suggesting that κ-opioid opioid receptor agonists act as potential therapeutic reagents for pruritus in HD patients. In Japan, a large-scale placebo-controlled study was performed to examine the efficacy and safety of oral nalfurafine hydrochloride for intractable pruritus in 337 HD patients. Two daily doses of 2.5 or 5 μg nalfurafine or placebo were orally administered for 2 weeks, and clinical responses were analyzed. The results showed that the mean decrease in the visual analog scale for pruritus from baseline was 22 mm in the 5 μg nalfurafine hydrochloride group (n=114) and 23 mm in the 2.5 μg group (n=112). These reductions were statistically significant compared with 13 mm, which is the mean decrease of visual analog scale in the placebo group (n=111), demonstrating that nalfurafine is an effective and safe drug for uremic pruritus in HD patients. Moreover, another open-label trial (n=145) examining the long-term effect of 5 μg oral nalfurafine revealed the maintenance of the antipruritic effect of nalfurafine for 52 weeks. In addition, on the basis of recent data showing κ-opioid receptor expression in the epidermis of atopic dermatitis and psoriasis, nalfurafine hydrochloride also can be potentially used for these two skin diseases.
Keywords: hemodialysis; nalfurafine hydrochloride; opioid κ-selective agonist; uremic pruritus.
Figures
References
-
- Nakai S, Hanafusa N, Masakane I, et al. An overview of regular dialysis treatment in Japan (as of December 31, 2012) Ther Apher Dial. 2014;18(6):535–602. - PubMed
-
- Pisoni RL, Wikström B, Elder SJ, et al. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2006;21(12):3495–3505. - PubMed
-
- Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69(9):1626–1632. - PubMed
-
- Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H. Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant. 2004;19(12):3137–3139. - PubMed
-
- Breneman DL, Cardone JS, Blumsack RF, Lather RM, Searle EA, Pollack VE. Topical capsaicin for treatment of hemodialysis-related pruritus. J Am Acad Dermatol. 1992;26(1):91–94. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials