Intracellular calcium dynamics in cortical microglia responding to focal laser injury in the PC::G5-tdT reporter mouse
- PMID: 26005403
- PMCID: PMC4424843
- DOI: 10.3389/fnmol.2015.00012
Intracellular calcium dynamics in cortical microglia responding to focal laser injury in the PC::G5-tdT reporter mouse
Abstract
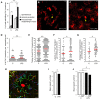
Microglia, the resident immune cells of the brain parenchyma, are highly responsive to tissue injury. Following cell damage, microglial processes redirect their motility from randomly scouting the extracellular space to specifically reaching toward the compromised tissue. While the cell morphology aspects of this defense mechanism have been characterized, the intracellular events underlying these responses remain largely unknown. Specifically, the role of intracellular Ca(2+) dynamics has not been systematically investigated in acutely activated microglia due to technical difficulty. Here we used live two-photon imaging of the mouse cortex ubiquitously expressing the genetically encoded Ca(2+) indicator GCaMP5G and fluorescent marker tdTomato in central nervous system microglia. We found that spontaneous Ca(2+) transients in microglial somas and processes were generally low (only 4% of all microglia showing transients within 20 min), but baseline activity increased about 8-fold when the animals were treated with LPS 12 h before imaging. When challenged with focal laser injury, an additional surge in Ca(2+) activity was observed in the somas and protruding processes. Notably, coherent and simultaneous Ca(2+) rises in multiple microglial cells were occasionally detected in LPS-treated animals. We show that Ca(2+) transients were pre-dominantly mediated via purinergic receptors. This work demonstrates the usefulness of genetically encoded Ca(2+) indicators for investigation of microglial physiology.
Keywords: GCaMP5G; GECI; PC::G5-tdT; calcium imaging; microglia; purinergic receptors.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

