Differential expression of sirtuins in the aging rat brain
- PMID: 26005404
- PMCID: PMC4424846
- DOI: 10.3389/fncel.2015.00167
Differential expression of sirtuins in the aging rat brain
Abstract
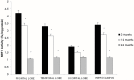
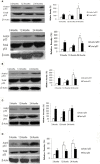
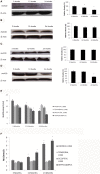
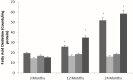
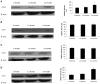
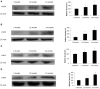
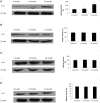
Although there are seven mammalian sirtuins (SIRT1-7), little is known about their expression in the aging brain. To characterize the change(s) in mRNA and protein expression of SIRT1-7 and their associated proteins in the brain of "physiologically" aged Wistar rats. We tested mRNA and protein expression levels of rat SIRT1-7, and the levels of associated proteins in the brain using RT-PCR and western blotting. Our data shows that SIRT1 expression increases with age, concurrently with increased acetylated p53 levels in all brain regions investigated. SIRT2 and FOXO3a protein levels increased only in the occipital lobe. SIRT3-5 expression declined significantly in the hippocampus and frontal lobe, associated with increases in superoxide and fatty acid oxidation levels, and acetylated CPS-1 protein expression, and a reduction in MnSOD level. While SIRT6 expression declines significantly with age acetylated H3K9 protein expression is increased throughout the brain. SIRT7 and Pol I protein expression increased in the frontal lobe. This study identifies previously unknown roles for sirtuins in regulating cellular homeostasis and healthy aging.
Keywords: aging; brain; longevity; p53; sirtuins.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

