Design of Pyridopyrazine-1,6-dione γ-Secretase Modulators that Align Potency, MDR Efflux Ratio, and Metabolic Stability
- PMID: 26005540
- PMCID: PMC4434474
- DOI: 10.1021/acsmedchemlett.5b00070
Design of Pyridopyrazine-1,6-dione γ-Secretase Modulators that Align Potency, MDR Efflux Ratio, and Metabolic Stability
Abstract
Herein we describe the design and synthesis of a series of pyridopyrazine-1,6-dione γ-secretase modulators (GSMs) for Alzheimer's disease (AD) that achieve good alignment of potency, metabolic stability, and low MDR efflux ratios, while also maintaining favorable physicochemical properties. Specifically, incorporation of fluorine enabled design of metabolically less liable lipophilic alkyl substituents to increase potency without compromising the sp(3)-character. The lead compound 21 (PF-06442609) displayed a favorable rodent pharmacokinetic profile, and robust reductions of brain Aβ42 and Aβ40 were observed in a guinea pig time-course experiment.
Keywords: Alzheimer’s disease; LipMetE; fluorine; gamma secretase modulators; lipophilic metabolism efficiency.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
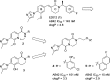
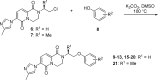
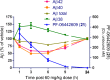
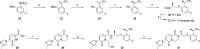
References
-
- Thies W.; Bleiler L. Alzheimer’s disease facts and figures. Alzheimers Dement. 2013, 9, 208–245. - PubMed
-
- Karran E.; Mercken M.; De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discovery 2011, 10, 698–712. - PubMed
-
- Bateman R. J.; Siemers E. R.; Mawuenyega K. G.; Wen G.; Browning K. R.; Sigurdson W. C.; Yarasheski K. E.; Friedrich S. W.; Demattos R. B.; May P. C.; Paul S. M.; Holtzman D. M. A γ-secretase inhibitor decreases amyloid-β production in the central nervous system. Ann. Neurol. 2009, 66, 48–54. - PMC - PubMed
-
- De Strooper B. Lessons from a failed γ-secretase Alzheimer trial. Cell 2014, 159, 721–726. - PubMed
-
- Coric V.; van Dyck C. H.; Salloway S.; Andreasen N.; Brody M.; Richter R. W.; Soininen H.; Thein S.; Shiovitz T.; Pilcher G.; Colby S.; Rollin L.; Dockens R.; Pachai C.; Portelius E.; Andreasson U.; Blennow K.; Soares H.; Albright C.; Feldman H. H.; Berman R. M. Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch. Neurol. 2012, 69, 1430–1440. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

