Preliminary documentation of the comparable efficacy of vitoss versus NanOss bioactive as bone graft expanders for posterior cervical fusion
- PMID: 26005578
- PMCID: PMC4431045
- DOI: 10.4103/2152-7806.156559
Preliminary documentation of the comparable efficacy of vitoss versus NanOss bioactive as bone graft expanders for posterior cervical fusion
Abstract
Background: Laminectomies with posterior cervical instrumented fusions often utilize bone graft expanders to supplement cervical lamina/iliac crest autograft/bone marrow aspirate (BMA). Here we compared posterior fusion rates utilizing two graft expanders; Vitoss (Orthovita, Malvern, PA, USA) vs. NanOss Bioactive (Regeneration Technologies Corporation [RTI: Alachua, FL, USA]).
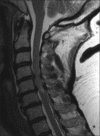
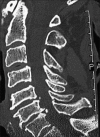
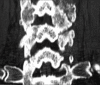
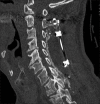
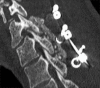
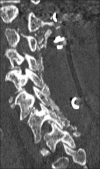
Methods: Two successive prospective cohorts of patients underwent 1-3 level laminectomies with 5-9 level posterior cervical fusions to address cervical spondylotic myelopathy (CSM) and/or ossification of the posterior longitudinal ligament (OPLL). The first cohort of 72 patients received Vitoss, while the second cohort or 20 patients received NanOss. Fusions were performed utilizing the Vertex/Rod/Eyelet System (Medtronic, Memphis, TN, USA) with braided titanium cables through the base of intact spinous processes (not lateral mass screws) cephalad and caudad to laminectomy defects. Fusion was documented by an independent neuroradiologist blinded to the study design, utilizing dynamic X-rays and two dimensional computed tomography (2D-CT) studies up to 6 months postoperatively, or until fusion or pseudarthrosis was confirmed at 1 year.
Results: Vitoss and NanOss resulted in comparable times to fusion: 5.65 vs. 5.35 months. Dynamic X-ray and CT-documented pseudarthrosis developed in 2 of 72 Vitoss patients at one postoperative year (e.g. bone graft resorbed secondary to early deep wound infections), while none occurred in the 20 patients receiving NanOss.
Conclusion: In this preliminary study combining cervical laminectomy/fusions, the time to fusion (5.65 vs. 5.35 months), pseudarthrosis (2.7% vs. 0%), and infection rates (2.7% vs. 0%) were nearly comparable sequentially utilizing Vitoss (72 patients) vs. NanOss (20 patients) as bone graft expanders.
Keywords: Autograft; NanOss bioactive; Vitoss; beta-tricalcium phosphate; fusion/pseudarthrosis rates; instrumented fusion; posterior cervical surgery.
Figures
Similar articles
-
Preliminary study showing safety/efficacy of nanoss bioactive versus vitoss as bone graft expanders for lumbar noninstrumented fusions.Surg Neurol Int. 2015 Jun 25;6(Suppl 10):S318-22. doi: 10.4103/2152-7806.159380. eCollection 2015. Surg Neurol Int. 2015. PMID: 26167369 Free PMC article.
-
High posterior cervical fusion rates with iliac autograft and Nanoss/bone marrow aspirate.Surg Neurol Int. 2017 Jul 20;8:152. doi: 10.4103/sni.sni_241_17. eCollection 2017. Surg Neurol Int. 2017. PMID: 28808601 Free PMC article.
-
High lumbar noninstrumented fusion rates using lamina autograft and Nanoss/bone marrow aspirate.Surg Neurol Int. 2017 Jul 20;8:153. doi: 10.4103/sni.sni_248_17. eCollection 2017. Surg Neurol Int. 2017. PMID: 28808602 Free PMC article.
-
Efficacy of different bone volume expanders for augmenting lumbar fusions.Surg Neurol. 2008 Jan;69(1):16-9; discussion 19. doi: 10.1016/j.surneu.2007.03.021. Surg Neurol. 2008. PMID: 18054607 Review.
-
Posterior plating of the cervical spine.J Spinal Disord. 1995 Apr;8(2):111-5. J Spinal Disord. 1995. PMID: 7606116 Review.
Cited by
-
Synthetic osteobiologics in spine surgery: a review.Turk J Med Sci. 2024 Oct 25;55(1):43-51. doi: 10.55730/1300-0144.5941. eCollection 2025. Turk J Med Sci. 2024. PMID: 40104322 Free PMC article. Review.
-
Autologous Stem Cells in Cervical Spine Fusion.Global Spine J. 2021 Jul;11(6):950-965. doi: 10.1177/2192568220948479. Epub 2020 Sep 23. Global Spine J. 2021. PMID: 32964752 Free PMC article.
-
Nursing review of cervical laminectomy and fusion.Surg Neurol Int. 2017 Dec 11;8:300. doi: 10.4103/sni.sni_243_17. eCollection 2017. Surg Neurol Int. 2017. PMID: 29296286 Free PMC article.
-
Synthetic Bone Graft Materials in Spine Fusion: Current Evidence and Future Trends.Int J Spine Surg. 2021 Apr;15(s1):104-112. doi: 10.14444/8058. Epub 2021 Apr 21. Int J Spine Surg. 2021. PMID: 34376499 Free PMC article.
-
Bone substitutes and expanders in Spine Surgery: A review of their fusion efficacies.Int J Spine Surg. 2016 Sep 22;10:33. doi: 10.14444/3033. eCollection 2016. Int J Spine Surg. 2016. PMID: 27909654 Free PMC article.
References
-
- Epstein NE. A preliminary study of the efficacy of Beta Tricalcium Phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006;19:424–9. - PubMed
-
- Epstein NE. An analysis of noninstrumented posterolateral lumbar fusions performed in predominantly geriatric patients using lamina autograft and beta tricalcium phosphate. Spine J. 2008;8:882–7. - PubMed
-
- Epstein NE. Beta tricalcium phosphate: Observation of use in 100 posterolateral lumbar instrumented fusions. Spine J. 2009;9:630–8. - PubMed
-
- Kaiser MG, Groff MW, Watters WC, 3rd, Ghogawala Z, Mummaneni PV, Dailey AT, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: Bone graft extenders and substitutes as an adjunct for lumbar fusion. J Neurosurg Spine. 2014;21:106–32. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
