Fibrinogen-Related Proteins in Tissue Repair: How a Unique Domain with a Common Structure Controls Diverse Aspects of Wound Healing
- PMID: 26005593
- PMCID: PMC4432967
- DOI: 10.1089/wound.2014.0599
Fibrinogen-Related Proteins in Tissue Repair: How a Unique Domain with a Common Structure Controls Diverse Aspects of Wound Healing
Abstract
Significance: Fibrinogen-related proteins (FRePs) comprise an intriguing collection of extracellular molecules, each containing a conserved fibrinogen-like globe (FBG). This group includes the eponymous fibrinogen as well as the tenascin, angiopoietin, and ficolin families. Many of these proteins are upregulated during tissue repair and exhibit diverse roles during wound healing. Recent Advances: An increasing body of evidence highlights the specific expression of a number of FRePs following tissue injury and infection. Upon induction, each FReP uses its FBG domain to mediate quite distinct effects that contribute to different stages of tissue repair, such as driving coagulation, pathogen detection, inflammation, angiogenesis, and tissue remodeling. Critical Issues: Despite a high degree of homology among FRePs, each contains unique sequences that enable their diversification of function. Comparative analysis of the structure and function of FRePs and precise mapping of regions that interact with a variety of ligands has started to reveal the underlying molecular mechanisms by which these proteins play very different roles using their common domain. Future Directions: Fibrinogen has long been used in the clinic as a synthetic matrix serving as a scaffold or a delivery system to aid tissue repair. Novel therapeutic strategies are now emerging that harness the use of other FRePs to improve wound healing outcomes. As we learn more about the underlying mechanisms by which each FReP contributes to the repair response, specific blockade, or indeed potentiation, of their function offers real potential to enable regulation of distinct processes during pathological wound healing.
Figures

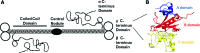


References
-
- Yee VC, et al. . Crystal structure of a 30 kDa C-terminal fragment from the gamma chain of human fibrinogen. Structure 1997;5:125–138 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

