Anatomic and physiological fundamentals for autologous breast reconstruction
- PMID: 26005644
- PMCID: PMC4409677
- DOI: 10.3978/j.issn.2227-684X.2015.04.01
Anatomic and physiological fundamentals for autologous breast reconstruction
Abstract
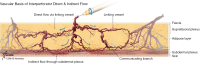
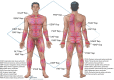
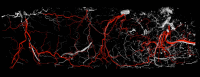
The success of autologous tissue transfer is reliant on adequate blood supply and as we endeavour to tailor our reconstructive options through our flap choices and design. Autologous breast reconstruction has made substantial progress over the years and the evolution of refinements over the last 30 years has allowed flaps to be based on specific perforators. The ultimate goal of breast reconstruction following mastectomy is to match optimal tissue replacement with minimal donor-site expenditure. In parallel surgeons will seek ways to ensure safe flap design and harvest while maintaining predictability and reliable tissue perfusion. Better understanding of the vascular anatomy and physiology of the cutaneous circulation of soft tissues, and that of patterns of blood flow from individual perforator has provided insight to advance perforator flap harvest and modifications in flap design. The aim of this article is to review the principles of blood supply and flap design exemplified through common flaps used in autologous breast reconstructive surgery, to better understand approaches for safe flap harvest and transfer of well perfused tissue.
Keywords: Autologous reconstruction; anatomy; blood supply; breast reconstruction; flap design; free flaps; pedicled flaps; perforasome; perforator flaps; vascular territory.
Figures




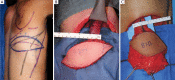
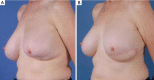
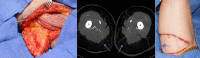


Similar articles
-
Pedicled perforator flaps in breast reconstruction: a new concept.Br J Plast Surg. 2004 Sep;57(6):531-9. doi: 10.1016/j.bjps.2004.04.015. Br J Plast Surg. 2004. PMID: 15308400
-
Unilateral Autologous Breast Reconstruction with Bi-pedicled, Conjoined Deep Inferior Epigastric Perforator Flaps.J Reconstr Microsurg. 2019 Feb;35(2):145-155. doi: 10.1055/s-0038-1668161. Epub 2018 Aug 15. J Reconstr Microsurg. 2019. PMID: 30112754
-
Experimental study of pedicled subcostal artery perforator (SCAP) flap: A new application in breast reconstruction.J Plast Reconstr Aesthet Surg. 2019 Aug;72(8):1299-1303. doi: 10.1016/j.bjps.2019.03.030. Epub 2019 Apr 16. J Plast Reconstr Aesthet Surg. 2019. PMID: 31103612
-
Dynamic InfraRed Thermography (DIRT) in DIEP-flap breast reconstruction: A review of the literature.Eur J Obstet Gynecol Reprod Biol. 2019 Nov;242:47-55. doi: 10.1016/j.ejogrb.2019.08.008. Epub 2019 Aug 23. Eur J Obstet Gynecol Reprod Biol. 2019. PMID: 31563818 Review.
-
Choice of flaps for breast reconstruction.Int J Clin Oncol. 2005 Oct;10(5):289-97. doi: 10.1007/s10147-005-0527-4. Int J Clin Oncol. 2005. PMID: 16247654 Review.
Cited by
-
Localization of perforators in autologous breast reconstruction with deep inferior epigastric perforator (DIEP)-flap-the indocyanine green angiography perspective.Gland Surg. 2023 Feb 28;12(2):129-133. doi: 10.21037/gs-22-745. Epub 2023 Jan 15. Gland Surg. 2023. PMID: 36915813 Free PMC article. No abstract available.
-
Improving the Safety of DIEP Flap Transplantation: Detailed Perforator Anatomy Study Using Preoperative CTA.J Pers Med. 2022 Apr 28;12(5):701. doi: 10.3390/jpm12050701. J Pers Med. 2022. PMID: 35629124 Free PMC article.
-
Revolutionizing Breast Reconstruction: The Rise of Hybrid Techniques.Medicina (Kaunas). 2025 Aug 9;61(8):1434. doi: 10.3390/medicina61081434. Medicina (Kaunas). 2025. PMID: 40870479 Free PMC article. Review.
-
Smartphone Thermal Imaging for Preoperative Perforator Mapping in Perforator Based Flaps.Cureus. 2024 Jan 6;16(1):e51755. doi: 10.7759/cureus.51755. eCollection 2024 Jan. Cureus. 2024. PMID: 38318547 Free PMC article.
References
-
- Levine SM, Lester ME, Fontenot B, et al. Perforator flap breast reconstruction after unsatisfactory implant reconstruction. Ann Plast Surg 2011;66:513-7. - PubMed
-
- Hamdi M, Andrades P, Thiessen F, et al. Is a second free flap still an option in a failed free flap breast reconstruction? Plast Reconstr Surg 2010;126:375-84. - PubMed
-
- Manchot C. eds. The cutaneous arteries of the human body. New York: Springer-Verlag, 1983.
-
- Cormack GC, Lamberty BG. Fasciocutaneous vessels. Their distribution on the trunk and limbs, and their clinical application in tissue transfer. Anat Clin 1984;6:121-31. - PubMed
-
- Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg 1987;40:113-41. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
