Haemocytes control stem cell activity in the Drosophila intestine
- PMID: 26005834
- PMCID: PMC4449816
- DOI: 10.1038/ncb3174
Haemocytes control stem cell activity in the Drosophila intestine
Abstract
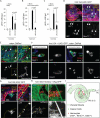
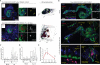
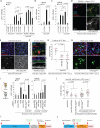
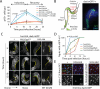
Coordination of stem cell activity with inflammatory responses is critical for regeneration and homeostasis of barrier epithelia. The temporal sequence of cell interactions during injury-induced regeneration is only beginning to be understood. Here we show that intestinal stem cells (ISCs) are regulated by macrophage-like haemocytes during the early phase of regenerative responses of the Drosophila intestinal epithelium. On tissue damage, haemocytes are recruited to the intestine and secrete the BMP homologue DPP, inducing ISC proliferation by activating the type I receptor Saxophone and the Smad homologue SMOX. Activated ISCs then switch their response to DPP by inducing expression of Thickveins, a second type I receptor that has previously been shown to re-establish ISC quiescence by activating MAD. The interaction between haemocytes and ISCs promotes infection resistance, but also contributes to the development of intestinal dysplasia in ageing flies. We propose that similar interactions influence pathologies such as inflammatory bowel disease and colorectal cancer in humans.
Figures
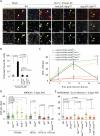
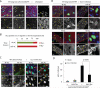
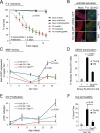
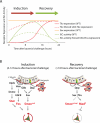
Comment in
-
Gut healing: haemocytes aid via Sax and Tkv jazzes it down.Nat Cell Biol. 2015 Jun;17(6):707-9. doi: 10.1038/ncb3178. Nat Cell Biol. 2015. PMID: 26022916
References
-
- Ferrandon D. The complementary facets of epithelial host defenses in the genetic model organism Drosophila melanogaster: from resistance to resilience. Current opinion in immunology. 2013;25:59–70. - PubMed
-
- Lemaitre B, Miguel-Aliaga I. The Digestive Tract of Drosophila melanogaster. Annu Rev Genet. 2013;47:377–404. - PubMed
-
- Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nature reviews. Microbiology. 2013;11:615–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

