S100A1 DNA-based Inotropic Therapy Protects Against Proarrhythmogenic Ryanodine Receptor 2 Dysfunction
- PMID: 26005840
- PMCID: PMC4817858
- DOI: 10.1038/mt.2015.93
S100A1 DNA-based Inotropic Therapy Protects Against Proarrhythmogenic Ryanodine Receptor 2 Dysfunction
Abstract
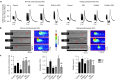
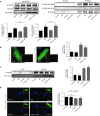
Restoring expression levels of the EF-hand calcium (Ca(2+)) sensor protein S100A1 has emerged as a key factor in reconstituting normal Ca(2+) handling in failing myocardium. Improved sarcoplasmic reticulum (SR) function with enhanced Ca(2+) resequestration appears critical for S100A1's cyclic adenosine monophosphate-independent inotropic effects but raises concerns about potential diastolic SR Ca(2+) leakage that might trigger fatal arrhythmias. This study shows for the first time a diminished interaction between S100A1 and ryanodine receptors (RyR2s) in experimental HF. Restoring this link in failing cardiomyocytes, engineered heart tissue and mouse hearts, respectively, by means of adenoviral and adeno-associated viral S100A1 cDNA delivery normalizes diastolic RyR2 function and protects against Ca(2+)- and β-adrenergic receptor-triggered proarrhythmogenic SR Ca(2+) leakage in vitro and in vivo. S100A1 inhibits diastolic SR Ca(2+) leakage despite aberrant RyR2 phosphorylation via protein kinase A and calmodulin-dependent kinase II and stoichiometry with accessory modulators such as calmodulin, FKBP12.6 or sorcin. Our findings demonstrate that S100A1 is a regulator of diastolic RyR2 activity and beneficially modulates diastolic RyR2 dysfunction. S100A1 interaction with the RyR2 is sufficient to protect against basal and catecholamine-triggered arrhythmic SR Ca(2+) leak in HF, combining antiarrhythmic potency with chronic inotropic actions.
Figures





Similar articles
-
S100A1's single cysteine is an indispensable redox switch for the protection against diastolic calcium waves in cardiomyocytes.Am J Physiol Heart Circ Physiol. 2024 Jul 1;327(1):H000. doi: 10.1152/ajpheart.00634.2023. Epub 2024 May 31. Am J Physiol Heart Circ Physiol. 2024. PMID: 38819384 Free PMC article.
-
Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure.Circ Res. 2005 Dec 9;97(12):1314-22. doi: 10.1161/01.RES.0000194329.41863.89. Epub 2005 Nov 3. Circ Res. 2005. PMID: 16269653
-
Defective Ca(2+) handling proteins regulation during heart failure.Physiol Res. 2011;60(1):27-37. doi: 10.33549/physiolres.931948. Epub 2010 Oct 15. Physiol Res. 2011. PMID: 20945956
-
Sarcoplasmic reticulum calcium leak and cardiac arrhythmias.Biochem Soc Trans. 2007 Nov;35(Pt 5):952-6. doi: 10.1042/BST0350952. Biochem Soc Trans. 2007. PMID: 17956253 Review.
-
Ca(2+)-handling proteins and heart failure: novel molecular targets?Curr Med Chem. 2003 Jun;10(11):967-81. doi: 10.2174/0929867033457656. Curr Med Chem. 2003. PMID: 12678683 Review.
Cited by
-
Role of ion channels in heart failure and channelopathies.Biophys Rev. 2018 Aug;10(4):1097-1106. doi: 10.1007/s12551-018-0442-3. Epub 2018 Jul 17. Biophys Rev. 2018. PMID: 30019205 Free PMC article. Review.
-
S100A1's single cysteine is an indispensable redox switch for the protection against diastolic calcium waves in cardiomyocytes.Am J Physiol Heart Circ Physiol. 2024 Jul 1;327(1):H000. doi: 10.1152/ajpheart.00634.2023. Epub 2024 May 31. Am J Physiol Heart Circ Physiol. 2024. PMID: 38819384 Free PMC article.
-
S100A1ct: A Synthetic Peptide Derived From S100A1 Protein Improves Cardiac Performance and Survival in Preclinical Heart Failure Models.Circulation. 2025 Feb 25;151(8):548-565. doi: 10.1161/CIRCULATIONAHA.123.066961. Epub 2024 Nov 21. Circulation. 2025. PMID: 39569500 Free PMC article.
-
Raf kinase inhibitor protein: lessons of a better way for β-adrenergic receptor activation in the heart.J Physiol. 2017 Jun 15;595(12):4073-4087. doi: 10.1113/JP274064. Epub 2017 May 23. J Physiol. 2017. PMID: 28444807 Free PMC article. Review.
-
Pathophysiological mechanism and therapeutic role of S100 proteins in cardiac failure: a systematic review.Heart Fail Rev. 2016 Sep;21(5):463-73. doi: 10.1007/s10741-016-9529-8. Heart Fail Rev. 2016. PMID: 26833319
References
-
- Bers, DM (2002). Cardiac excitation-contraction coupling. Nature 415: 198–205. - PubMed
-
- Bers, DM (2014). Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu Rev Physiol 76: 107–127. - PubMed
-
- Morgan, JP, Erny, RE, Allen, PD, Grossman, W and Gwathmey, JK (1990). Abnormal intracellular calcium handling, a major cause of systolic and diastolic dysfunction in ventricular myocardium from patients with heart failure. Circulation 81(2 Suppl): III21–III32. - PubMed
-
- Farr, MA and Basson, CT (2004). Sparking the failing heart. N Engl J Med 351: 185–187. - PubMed
-
- Katz, AM (1986). Potential deleterious effects of inotropic agents in the therapy of chronic heart failure. Circulation 73(3 Pt 2): III184–III190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

