The Cyclophilin A-CD147 complex promotes the proliferation and homing of multiple myeloma cells
- PMID: 26005854
- PMCID: PMC4567046
- DOI: 10.1038/nm.3867
The Cyclophilin A-CD147 complex promotes the proliferation and homing of multiple myeloma cells
Erratum in
-
Author Correction: The Cyclophilin A-CD147 complex promotes the proliferation and homing of multiple myeloma cells.Nat Med. 2024 Apr;30(4):1210. doi: 10.1038/s41591-024-02820-2. Nat Med. 2024. PMID: 38273148 No abstract available.
-
Author Correction: The Cyclophilin A-CD147 complex promotes the proliferation and homing of multiple myeloma cells.Nat Med. 2024 Aug;30(8):2372. doi: 10.1038/s41591-024-03171-8. Nat Med. 2024. PMID: 38969881 No abstract available.
Abstract
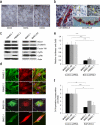
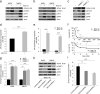
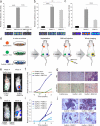
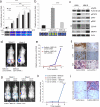
B cell malignancies frequently colonize the bone marrow. The mechanisms responsible for this preferential homing are incompletely understood. Here we studied multiple myeloma (MM) as a model of a terminally differentiated B cell malignancy that selectively colonizes the bone marrow. We found that extracellular CyPA (eCyPA), secreted by bone marrow endothelial cells (BMECs), promoted the colonization and proliferation of MM cells in an in vivo scaffold system via binding to its receptor, CD147, on MM cells. The expression and secretion of eCyPA by BMECs was enhanced by BCL9, a Wnt-β-catenin transcriptional coactivator that is selectively expressed by these cells. eCyPA levels were higher in bone marrow serum than in peripheral blood in individuals with MM, and eCyPA-CD147 blockade suppressed MM colonization and tumor growth in the in vivo scaffold system. eCyPA also promoted the migration of chronic lymphocytic leukemia and lymphoplasmacytic lymphoma cells, two other B cell malignancies that colonize the bone marrow and express CD147. These findings suggest that eCyPA-CD147 signaling promotes the bone marrow homing of B cell malignancies and offer a compelling rationale for exploring this axis as a therapeutic target for these malignancies.
Figures


Comment in
-
Multiple myeloma: Mama, I'm coming home.Nat Rev Cancer. 2015 Jul;15(7):384. doi: 10.1038/nrc3980. Epub 2015 Jun 18. Nat Rev Cancer. 2015. PMID: 26084392 No abstract available.
Similar articles
-
CD147-Cyclophilin a Interactions Promote Proliferation and Survival of Cutaneous T-Cell Lymphoma.Int J Mol Sci. 2021 Jul 23;22(15):7889. doi: 10.3390/ijms22157889. Int J Mol Sci. 2021. PMID: 34360654 Free PMC article.
-
The binding of extracellular cyclophilin A to ACE2 and CD147 triggers psoriasis-like inflammation.J Autoimmun. 2024 Sep;148:103293. doi: 10.1016/j.jaut.2024.103293. Epub 2024 Aug 2. J Autoimmun. 2024. PMID: 39096717
-
miR-30-5p functions as a tumor suppressor and novel therapeutic tool by targeting the oncogenic Wnt/β-catenin/BCL9 pathway.Cancer Res. 2014 Mar 15;74(6):1801-13. doi: 10.1158/0008-5472.CAN-13-3311-T. Epub 2014 Mar 5. Cancer Res. 2014. PMID: 24599134 Free PMC article.
-
Cyclophilin A/CD147 Interaction: A Promising Target for Anticancer Therapy.Int J Mol Sci. 2022 Aug 19;23(16):9341. doi: 10.3390/ijms23169341. Int J Mol Sci. 2022. PMID: 36012604 Free PMC article. Review.
-
Diagnostic and clinical considerations in concomitant bone marrow involvement by plasma cell myeloma and chronic lymphocytic leukemia/monoclonal B-cell lymphocytosis: a series of 15 cases and review of literature.Arch Pathol Lab Med. 2013 Apr;137(4):503-17. doi: 10.5858/arpa.2011-0696-OA. Arch Pathol Lab Med. 2013. PMID: 23544940 Review.
Cited by
-
Metabolic Function and Therapeutic Potential of CD147 for Hematological Malignancies: An Overview.Int J Mol Sci. 2024 Aug 23;25(17):9178. doi: 10.3390/ijms25179178. Int J Mol Sci. 2024. PMID: 39273126 Free PMC article. Review.
-
Progress and Paradigms in Multiple Myeloma.Clin Cancer Res. 2016 Nov 15;22(22):5419-5427. doi: 10.1158/1078-0432.CCR-16-0625. Clin Cancer Res. 2016. PMID: 28151709 Free PMC article.
-
Quantitative expression of Ikaros, IRF4, and PSMD10 proteins predicts survival in VRD-treated patients with multiple myeloma.Blood Adv. 2020 Dec 8;4(23):6023-6033. doi: 10.1182/bloodadvances.2020002711. Blood Adv. 2020. PMID: 33284947 Free PMC article.
-
Integrated transcriptomic and metabolomic analysis reveals the effects of EMMPRIN on nucleotide metabolism and 1C metabolism in AS mouse BMDMs.Front Mol Biosci. 2025 Mar 7;11:1460186. doi: 10.3389/fmolb.2024.1460186. eCollection 2024. Front Mol Biosci. 2025. PMID: 40125455 Free PMC article.
-
Prognostic Significance of Tumor-Associated Inflammation in Renal Cell Carcinoma.Bull Exp Biol Med. 2024 Jan;176(3):382-385. doi: 10.1007/s10517-024-06028-9. Epub 2024 Feb 10. Bull Exp Biol Med. 2024. PMID: 38340200
References
-
- Muller-Hermelink HK ME, Catovsky D, Campo E, Harris NL, Stein H. Chronic lymphocytic leukemia/small lymphocytic lymphoma. World Health Organization Classification of Tumor, Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. 2008:180–182.
-
- Anderson KC. Oncogenomics to target myeloma in the bone marrow microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:1225–1233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

