Prolonged and tunable residence time using reversible covalent kinase inhibitors
- PMID: 26006010
- PMCID: PMC4472506
- DOI: 10.1038/nchembio.1817
Prolonged and tunable residence time using reversible covalent kinase inhibitors
Abstract
Drugs with prolonged on-target residence times often show superior efficacy, yet general strategies for optimizing drug-target residence time are lacking. Here we made progress toward this elusive goal by targeting a noncatalytic cysteine in Bruton's tyrosine kinase (BTK) with reversible covalent inhibitors. Using an inverted orientation of the cysteine-reactive cyanoacrylamide electrophile, we identified potent and selective BTK inhibitors that demonstrated biochemical residence times spanning from minutes to 7 d. An inverted cyanoacrylamide with prolonged residence time in vivo remained bound to BTK for more than 18 h after clearance from the circulation. The inverted cyanoacrylamide strategy was further used to discover fibroblast growth factor receptor (FGFR) kinase inhibitors with residence times of several days, demonstrating the generalizability of the approach. Targeting of noncatalytic cysteines with inverted cyanoacrylamides may serve as a broadly applicable platform that facilitates 'residence time by design', the ability to modulate and improve the duration of target engagement in vivo.
Figures



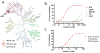

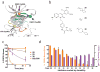
Comment in
-
Rational drug design: Tuning kinase inhibitor residence time.Nat Rev Drug Discov. 2015 Jul;14(7):457. doi: 10.1038/nrd4673. Epub 2015 Jun 19. Nat Rev Drug Discov. 2015. PMID: 26091268 No abstract available.
References
-
- Copeland RA, Pompliano DL, Meek TD. Drug-target residence time and its implications for for lead optimization. Nat Rev Drug Disc. 2006;5:730–739. - PubMed
-
- Copeland RA. The dynamics of drug-target interactions: drug-target residence time and its impact on efficacy and safety. Expert Opin Drug Disc. 2010;5:305–310. - PubMed
-
- Guo D, Hillger JM, IJzerman AP, Heitman LH. Drug-target residence time- a case for G protein-coupled receptors. Med Res Rev. 2014;34:856–892. - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/250230078
- PubChem-Substance/250230079
- PubChem-Substance/250230080
- PubChem-Substance/250230081
- PubChem-Substance/250230082
- PubChem-Substance/250230083
- PubChem-Substance/250230084
- PubChem-Substance/250230085
- PubChem-Substance/250230086
- PubChem-Substance/250230087
- PubChem-Substance/250230088
- PubChem-Substance/250230089
- PubChem-Substance/250230090
- PubChem-Substance/250230091
- PubChem-Substance/250230092
- PubChem-Substance/250230093
- PubChem-Substance/250230094
- PubChem-Substance/250230095
- PubChem-Substance/250230096
- PubChem-Substance/250230097
- PubChem-Substance/250230098
- PubChem-Substance/250230099
- PubChem-Substance/250230100
- PubChem-Substance/250230101
- PubChem-Substance/250230102
- PubChem-Substance/250230103
- PubChem-Substance/250230104
- PubChem-Substance/250230105
- PubChem-Substance/250230106
- PubChem-Substance/250230107
- PubChem-Substance/250230108
- PubChem-Substance/250230109
- PubChem-Substance/250230110
- PubChem-Substance/250230111
- PubChem-Substance/250230112
- PubChem-Substance/250230113
- PubChem-Substance/250230114
- PubChem-Substance/250230115
- PubChem-Substance/250230116
- PubChem-Substance/250230117
- PubChem-Substance/250230118
- PubChem-Substance/250230119
- PubChem-Substance/250230120
- PubChem-Substance/250230121
- PubChem-Substance/250230122
- PubChem-Substance/250230123
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

