Convergence of biological nitration and nitrosation via symmetrical nitrous anhydride
- PMID: 26006011
- PMCID: PMC4472503
- DOI: 10.1038/nchembio.1814
Convergence of biological nitration and nitrosation via symmetrical nitrous anhydride
Abstract
The current perspective holds that the generation of secondary signaling mediators from nitrite (NO2(-)) requires acidification to nitrous acid (HNO2) or metal catalysis. Herein, the use of stable isotope-labeled NO2(-) and LC-MS/MS analysis of products reveals that NO2(-) also participates in fatty acid nitration and thiol S-nitrosation at neutral pH. These reactions occur in the absence of metal centers and are stimulated by autoxidation of nitric oxide ((•)NO) via the formation of symmetrical dinitrogen trioxide (nitrous anhydride, symN2O3). Although theoretical models have predicted physiological symN2O3 formation, its generation is now demonstrated in aqueous reaction systems, cell models and in vivo, with the concerted reactions of (•)NO and NO2(-) shown to be critical for symN2O3 formation. These results reveal new mechanisms underlying the NO2(-) propagation of (•)NO signaling and the regulation of both biomolecule function and signaling network activity via NO2(-)-dependent nitrosation and nitration reactions.
Figures
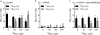


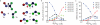

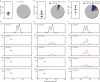
References
-
- Lancaster JR., Jr. Protein cysteine thiol nitrosation: maker or marker of reactive nitrogen species-induced nonerythroid cellular signaling? Nitric Oxide. 2008;19:68–72. - PubMed
-
- Moller MN, et al. Membrane “lens” effect: focusing the formation of reactive nitrogen oxides from the *NO/O2 reaction. Chem Res Toxicol. 2007;20:709–714. - PubMed
-
- Nedospasov AA. Is N2O3 the main nitrosating intermediate in aerated nitric oxide (NO) solutions in vivo? If so, where, when, and which one? J Biochem Mol Toxicol. 2002;16:109–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

