The hepatocyte growth factor receptor as a potential therapeutic target for dedifferentiated liposarcoma
- PMID: 26006023
- PMCID: PMC4520775
- DOI: 10.1038/labinvest.2015.62
The hepatocyte growth factor receptor as a potential therapeutic target for dedifferentiated liposarcoma
Abstract
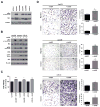
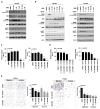
Dedifferentiated liposarcomas (DDLPS) are highly resistant to conventional chemo- and radiotherapies, with surgical resection remaining the classic treatment strategy; therefore, there is a pressing need for novel anti-DDLPS-targeted chemotherapeutics. Hepatocyte growth factor receptor (Met) expression is elevated in DDLPS, but the functional role of Met signaling in this disease is not known. We found that the in vitro stimulation of DDLPS cells with hepatocyte growth factor (HGF) elevated the degree of PI3K/AKT and MAPK pathway signaling, and that pro-tumorigenic phenotypes such as cell proliferation, invasion, and migration were significantly enhanced. Conversely, Met knockdown using shRNA-mediated interference decreased HGF-induced Met signaling, the invasive and migratory nature of DDLPS cells in vitro, and the tumorigenicity of DDLPS cells in vivo. These data strongly support the role for Met as a DDLPS therapeutic target. To that end, using EMD1214063, an ATP-competitive kinase inhibitor that targets Met more specifically than other kinases, inhibited Met-dependent signaling, reduced the oncogenicity of DDLPS cells in vitro, and significantly increased the survival of nude mice bearing subcutaneous DDLPS xenografts. These findings support further investigations of HGF-induced Met signaling inhibition in DDLPS, as a potential strategy to enhance clinical outcomes for this disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hoffman A, Lazar AJ, Pollock RE, Lev D. Drug Resist Updat. 1. Vol. 14. Elsevier Ltd; 2011. Feb, New frontiers in the treatment of liposarcoma, a therapeutically resistant malignant cohort; pp. 52–66. - PubMed
-
- Singer S, Socci ND, Ambrosini G, Sambol E, Decarolis P, Wu Y, et al. Gene expression profiling of liposarcoma identifies distinct biological types/subtypes and potential therapeutic targets in well-differentiated and dedifferentiated liposarcoma. Cancer Res. 2007 Jul 15;67(14):6626–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

