Mechanotransduction via TRPV4 regulates inflammation and differentiation in fetal mouse distal lung epithelial cells
- PMID: 26006045
- PMCID: PMC4446903
- DOI: 10.1186/s12931-015-0224-4
Mechanotransduction via TRPV4 regulates inflammation and differentiation in fetal mouse distal lung epithelial cells
Abstract
Background: Mechanical ventilation plays a central role in the injury of premature lungs. However, the mechanisms by which mechanical signals trigger an inflammatory cascade to promote lung injury are not well-characterized. Transient receptor potential vanilloid 4 (TRPV4), a calcium-permeable mechanoreceptor channel has been shown to be a major determinant of ventilator-induced acute lung injury in adult models. However, the role of these channels as modulators of inflammation in immature lungs is unknown. In this study, we tested the hypothesis that TRPV4 channels are important mechanotransducers in fetal lung injury.
Methods: Expression of TRPV4 in the mouse fetal lung was investigated by immunohistochemistry, Western blot and qRT-PCR. Isolated fetal epithelial cells were exposed to mechanical stimulation using the Flexcell Strain Unit and inflammation and differentiation were analyzed by ELISA and SP-C mRNA, respectively.
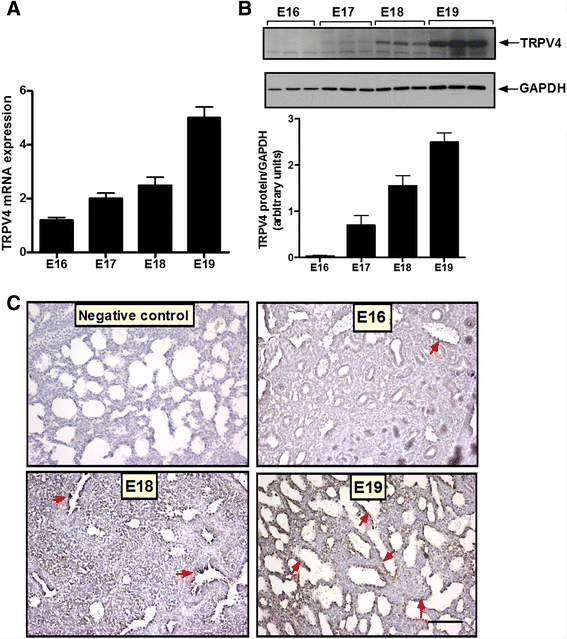
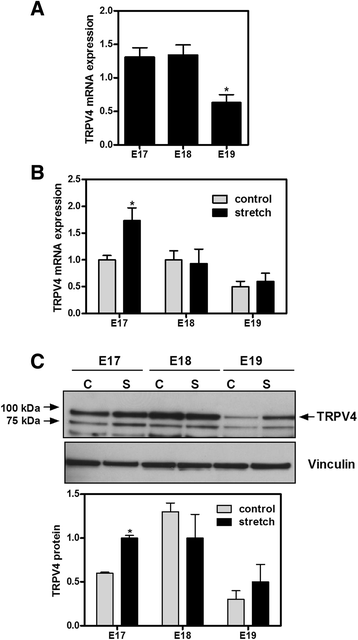
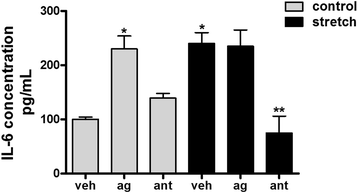
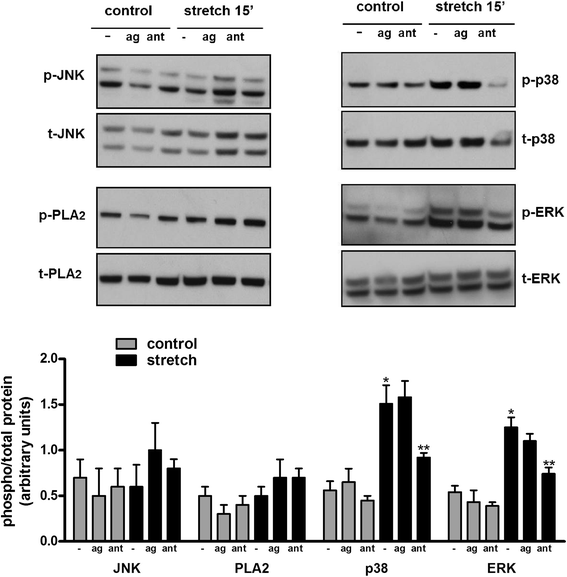
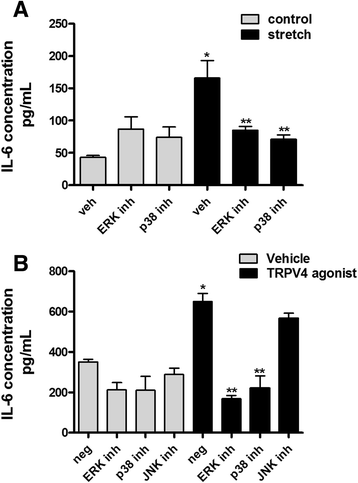
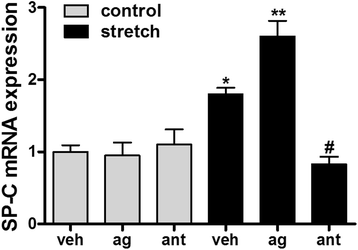
Results: TRPV4 is developmentally regulated in the fetal mouse lung; it is expressed in the lung epithelium and increases with advanced gestation. In contrast, in isolated epithelial cells, TRPV4 expression is maximal at E17-E18 of gestation. Mechanical stretch increases TRPV4 in isolated fetal epithelial cells only during the canalicular stage of lung development. Using the TRPV4 agonist GSK1016790A, the antagonist HC-067047, and the cytokine IL-6 as a marker of inflammation, we observed that TRPV4 regulates release of IL-6 via p38 and ERK pathways. Interestingly, stretch-induced differentiation of fetal epithelial cells was also modulated by TRPV4.
Conclusion: These studies demonstrate that TRPV4 may play an important role in the transduction of mechanical signals in the fetal lung epithelium by modulating not only inflammation but also the differentiation of fetal epithelial cells.
Figures
Similar articles
-
TRPV4-A Missing Link Between Mechanosensation and Immunity.Front Immunol. 2020 Mar 10;11:413. doi: 10.3389/fimmu.2020.00413. eCollection 2020. Front Immunol. 2020. PMID: 32210976 Free PMC article. Review.
-
Transient Receptor Potential Vanilloid 4 and Serum Glucocorticoid-regulated Kinase 1 Are Critical Mediators of Lung Injury in Overventilated Mice In Vivo.Anesthesiology. 2017 Feb;126(2):300-311. doi: 10.1097/ALN.0000000000001443. Anesthesiology. 2017. PMID: 27861175
-
Polymodal Sensory Transduction in Mouse Corneal Epithelial Cells.Invest Ophthalmol Vis Sci. 2020 Apr 9;61(4):2. doi: 10.1167/iovs.61.4.2. Invest Ophthalmol Vis Sci. 2020. PMID: 32271891 Free PMC article.
-
Acid inhibits TRPV4-mediated Ca²⁺ influx in mouse esophageal epithelial cells.Neurogastroenterol Motil. 2011 Nov;23(11):1020-8, e497. doi: 10.1111/j.1365-2982.2011.01767.x. Epub 2011 Aug 23. Neurogastroenterol Motil. 2011. PMID: 21883699
-
Role of Nonneuronal TRPV4 Signaling in Inflammatory Processes.Adv Pharmacol. 2017;79:117-139. doi: 10.1016/bs.apha.2017.03.002. Epub 2017 Apr 24. Adv Pharmacol. 2017. PMID: 28528666 Review.
Cited by
-
Mechanical stretch promotes sustained proliferation and inflammation in developing human airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2025 Aug 1;329(2):L296-L306. doi: 10.1152/ajplung.00070.2025. Epub 2025 Jul 16. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 40668642 Free PMC article.
-
TRPV4 Inhibition and CRISPR-Cas9 Knockout Reduce Inflammation Induced by Hyperphysiological Stretching in Human Annulus Fibrosus Cells.Cells. 2020 Jul 21;9(7):1736. doi: 10.3390/cells9071736. Cells. 2020. PMID: 32708074 Free PMC article.
-
TRPV4-A Missing Link Between Mechanosensation and Immunity.Front Immunol. 2020 Mar 10;11:413. doi: 10.3389/fimmu.2020.00413. eCollection 2020. Front Immunol. 2020. PMID: 32210976 Free PMC article. Review.
-
The TRPV4-TAZ mechanotransduction signaling axis in matrix stiffness- and TGFβ1-induced epithelial-mesenchymal transition.Cell Mol Bioeng. 2019 Apr;12(2):139-152. doi: 10.1007/s12195-018-00565-w. Epub 2018 Dec 4. Cell Mol Bioeng. 2019. PMID: 31681446 Free PMC article.
-
TRPV4 Channel in Neurological Disease: from Molecular Mechanisms to Therapeutic Potential.Mol Neurobiol. 2025 Mar;62(3):3877-3891. doi: 10.1007/s12035-024-04518-5. Epub 2024 Sep 28. Mol Neurobiol. 2025. PMID: 39333347 Free PMC article. Review.
References
-
- Kitterman JA. The effects of mechanical forces on fetal lung growth. Clin Perinatol. 1996;23:727–40. - PubMed
-
- Liggins GC. Growth of the fetal lung. J Dev Physiol. 1984;6:237–48. - PubMed
-
- Scarpelli EM, Condorelli S, Cosmi EV. Lamb fetal pulmonary fluid. I. Validation and significance of method for determination of volume and volume change. Pediatr Res. 1975;9:190–5. - PubMed
-
- Harding R. Fetal breathing movements. In: Crystal RG, West JB, Banes PJ, Weiber ER, editors. The Lung: Scientific Fountations. 2. Lippincott-Raven: Philadelphia; 1997. pp. 2093–104.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

