Intravital FRET: Probing Cellular and Tissue Function in Vivo
- PMID: 26006244
- PMCID: PMC4463726
- DOI: 10.3390/ijms160511713
Intravital FRET: Probing Cellular and Tissue Function in Vivo
Abstract
The development of intravital Förster Resonance Energy Transfer (FRET) is required to probe cellular and tissue function in the natural context: the living organism. Only in this way can biomedicine truly comprehend pathogenesis and develop effective therapeutic strategies. Here we demonstrate and discuss the advantages and pitfalls of two strategies to quantify FRET in vivo-ratiometrically and time-resolved by fluorescence lifetime imaging-and show their concrete application in the context of neuroinflammation in adult mice.
Keywords: fluorescence lifetime imaging; genetically encoded calcium indicators; intravital FRET; multi-photon microscopy.
Figures
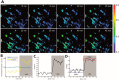
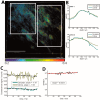

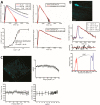
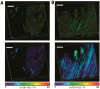
References
-
- Roszik J., Toth G., Szollosi J., Vereb G. Validating pharmacological disruption of protein-protein interactions by acceptor photobleaching FRET imaging. Methods Mol. Biol. 2013;986:165–178. - PubMed
-
- Kumar S., Alibhai D., Margineanu A., Laine R., Kennedy G., McGinty J., Warren S., Kelly D., Alexandrov Y., Munro I., et al. FLIM FRET technology for drug discovery: Automated multiwell-plate high-content analysis, multiplexed readouts and application in situ. Chemphyschem. 2011;12:609–626. doi: 10.1002/cphc.201000874. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

