Synthesis, Antifungal Activity and Structure-Activity Relationships of Novel 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic Acid Amides
- PMID: 26007171
- PMCID: PMC6272562
- DOI: 10.3390/molecules20058395
Synthesis, Antifungal Activity and Structure-Activity Relationships of Novel 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic Acid Amides
Abstract
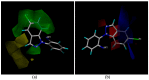
A series of novel 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid amides were synthesized and their activities were tested against seven phytopathogenic fungi by an in vitro mycelia growth inhibition assay. Most of them displayed moderate to excellent activities. Among them N-(2-(5-bromo-1H-indazol-1-yl)phenyl)-3-(difluoro-methyl)-1-methyl-1H-pyrazole-4-carboxamide (9m) exhibited higher antifungal activity against the seven phytopathogenic fungi than boscalid. Topomer CoMFA was employed to develop a three-dimensional quantitative structure-activity relationship model for the compounds. In molecular docking, the carbonyl oxygen atom of 9m could form hydrogen bonds towards the hydroxyl of TYR58 and TRP173 on SDH.
Keywords: CoMFA; SDHIs; fungicidal activity; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

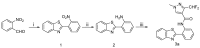

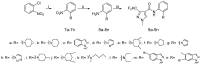
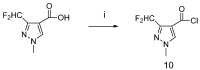

Similar articles
-
Synthesis, antifungal activity and QSAR of some novel carboxylic acid amides.Molecules. 2015 Mar 4;20(3):4071-87. doi: 10.3390/molecules20034071. Molecules. 2015. PMID: 25749678 Free PMC article.
-
Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1,3,4-thiadiazol-2-yl)cyclopropanecarboxamides.Eur J Med Chem. 2009 Jul;44(7):2782-6. doi: 10.1016/j.ejmech.2009.01.012. Epub 2009 Jan 22. Eur J Med Chem. 2009. PMID: 19246128
-
Synthesis of pyrazole-4-carboxamides as potential fungicide candidates.Mol Divers. 2021 Nov;25(4):2379-2388. doi: 10.1007/s11030-020-10127-w. Epub 2020 Jul 30. Mol Divers. 2021. PMID: 32734588
-
Evaluation of the antifungal activity of novel bis-pyrazole carboxamide derivatives and preliminary investigation of the mechanism.Bioorg Chem. 2024 Dec;153:107779. doi: 10.1016/j.bioorg.2024.107779. Epub 2024 Sep 1. Bioorg Chem. 2024. PMID: 39236583
-
In Pursuit of Lead Innovation: Pharmaceutically Important and Distinct Amide-Free Succinate Dehydrogenase Inhibitors.J Med Chem. 2025 Jan 23;68(2):1051-1067. doi: 10.1021/acs.jmedchem.4c02757. Epub 2025 Jan 1. J Med Chem. 2025. PMID: 39742458 Review.
Cited by
-
Novel Pyrazole-Hydrazone Derivatives Containing an Isoxazole Moiety: Design, Synthesis, and Antiviral Activity.Molecules. 2018 Jul 20;23(7):1798. doi: 10.3390/molecules23071798. Molecules. 2018. PMID: 30037021 Free PMC article.
-
Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review.Molecules. 2018 Jan 12;23(1):134. doi: 10.3390/molecules23010134. Molecules. 2018. PMID: 29329257 Free PMC article. Review.
-
New insides into chimeric and hybrid azines derivatives with antifungal activity.Future Med Chem. 2024;16(11):1163-1180. doi: 10.1080/17568919.2024.2351288. Epub 2024 May 28. Future Med Chem. 2024. PMID: 38916566 Free PMC article. Review.
-
Synthesis Candidates Herbicide Through Optimization Quinclorac Containing 3-Methyl-1H-pyrazol-5-yl.Front Chem. 2021 Apr 14;9:647472. doi: 10.3389/fchem.2021.647472. eCollection 2021. Front Chem. 2021. PMID: 33937195 Free PMC article.
-
Design, Synthesis, DFT Study and Antifungal Activity of Pyrazolecarboxamide Derivatives.Molecules. 2016 Jan 8;21(1):68. doi: 10.3390/molecules21010068. Molecules. 2016. PMID: 26760990 Free PMC article.
References
-
- Schmidt A., Beutler A., Snovydovych B. Recent Advances in the Chemistry of Indazoles. Eur. J. Org. Chem. 2008;24:4073–4095. doi: 10.1002/ejoc.200800227. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

