Synthesis and Biological Evaluation of N-Alkyl-3-(alkylamino)-pyrazine-2-carboxamides
- PMID: 26007174
- PMCID: PMC6272192
- DOI: 10.3390/molecules20058687
Synthesis and Biological Evaluation of N-Alkyl-3-(alkylamino)-pyrazine-2-carboxamides
Abstract
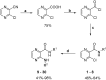
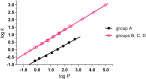
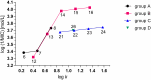
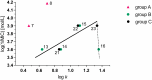
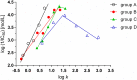
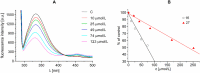
A series of N-alkyl-3-(alkylamino)pyrazine-2-carboxamides and their N-alkyl-3-chloropyrazine-2-carboxamide precursors were prepared. All compounds were characterized by analytical methods and tested for antimicrobial and antiviral activity. The antimycobacterial MIC values against Mycobacterium tuberculosis H37Rv of the most effective compounds, 3-(hexylamino)-, 3-(heptylamino)- and 3-(octylamino)-N-methyl-pyrazine-2-carboxamides 14‒16, was 25 μg/mL. The compounds inhibited photosystem 2 photosynthetic electron transport (PET) in spinach chloroplasts. This activity was strongly connected with the lipophilicity of the compounds. For effective PET inhibition longer alkyl chains in the 3-(alkylamino) substituent in the N-alkyl-3-(alkylamino)pyrazine-2-carboxamide molecule were more favourable than two shorter alkyl chains.
Keywords: alkylation; aminodehalogenation; antimycobacterial activity; inhibition of photosynthetic electron transport; pyrazinamide; pyrazine; structure-activity relationships.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Synthesis and antimycobacterial evaluation of N-substituted 3-aminopyrazine-2,5-dicarbonitriles.Bioorg Med Chem Lett. 2012 Feb 15;22(4):1598-601. doi: 10.1016/j.bmcl.2011.12.129. Epub 2012 Jan 3. Bioorg Med Chem Lett. 2012. PMID: 22281187
-
New potentially active pyrazinamide derivatives synthesized under microwave conditions.Molecules. 2014 Jul 3;19(7):9318-38. doi: 10.3390/molecules19079318. Molecules. 2014. PMID: 24995919 Free PMC article.
-
Synthesis, antimycobacterial, antifungal and photosynthesis-inhibiting activity of chlorinated N-phenylpyrazine-2-carboxamides.Molecules. 2010 Nov 26;15(12):8567-81. doi: 10.3390/molecules15128567. Molecules. 2010. PMID: 21116226 Free PMC article.
-
Synthesis and antimycobacterial evaluation of N-substituted 5-chloropyrazine-2-carboxamides.Bioorg Med Chem Lett. 2013 Jun 15;23(12):3589-91. doi: 10.1016/j.bmcl.2013.04.021. Epub 2013 Apr 21. Bioorg Med Chem Lett. 2013. PMID: 23659859
-
Antimycobacterial evaluation of pyrazinoic acid reversible derivatives.Curr Pharm Des. 2011;17(32):3506-14. doi: 10.2174/138161211798194477. Curr Pharm Des. 2011. PMID: 22074423 Review.
Cited by
-
Derivatives of 3-Aminopyrazine-2-carboxamides: Synthesis, Antimicrobial Evaluation, and in Vitro Cytotoxicity.Molecules. 2019 Mar 28;24(7):1212. doi: 10.3390/molecules24071212. Molecules. 2019. PMID: 30925695 Free PMC article.
-
Synthesis and Antifungal Screening of 2-{[1-(5-Alkyl/arylalkylpyrazin-2-yl)ethylidene]hydrazono}-1,3-thiazolidin-4-ones.Molecules. 2016 Nov 23;21(11):1592. doi: 10.3390/molecules21111592. Molecules. 2016. PMID: 27886119 Free PMC article.
-
Effects of Novel Photosynthetic Inhibitor [CuL2]Br2 Complex on Photosystem II Activity in Spinach.Cells. 2022 Aug 28;11(17):2680. doi: 10.3390/cells11172680. Cells. 2022. PMID: 36078088 Free PMC article.
-
Structure activity relationship of pyrazinoic acid analogs as potential antimycobacterial agents.Bioorg Med Chem. 2022 Nov 15;74:117046. doi: 10.1016/j.bmc.2022.117046. Epub 2022 Oct 7. Bioorg Med Chem. 2022. PMID: 36228522 Free PMC article.
-
3-Substituted N-Benzylpyrazine-2-carboxamide Derivatives: Synthesis, Antimycobacterial and Antibacterial Evaluation.Molecules. 2017 Mar 21;22(3):495. doi: 10.3390/molecules22030495. Molecules. 2017. PMID: 28335571 Free PMC article.
References
-
- Miniyar P.B., Murumkar P.R., Patil P.S., Barmade M.A., Bothara K.G. Unequivocal role of pyrazine ring in medicinally important compounds: A review. Mini Rev. Med. Chem. 2013;13:1607–1625. - PubMed
-
- Dolezal M., Zitko J. Pyrazine derivatives: A patent review (June 2012-present) Expert Opin. Ther. Pat. 2014;25:33–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

