Recent advances in click chemistry applied to dendrimer synthesis
- PMID: 26007183
- PMCID: PMC6272213
- DOI: 10.3390/molecules20059263
Recent advances in click chemistry applied to dendrimer synthesis
Abstract
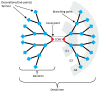
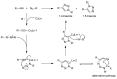
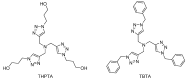
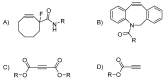
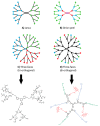
Dendrimers are monodisperse polymers grown in a fractal manner from a central point. They are poised to become the cornerstone of nanoscale devices in several fields, ranging from biomedicine to light-harvesting. Technical difficulties in obtaining these molecules has slowed their transfer from academia to industry. In 2001, the arrival of the "click chemistry" concept gave the field a major boost. The flagship reaction, a modified Hüisgen cycloaddition, allowed researchers greater freedom in designing and building dendrimers. In the last five years, advances in click chemistry saw a wider use of other click reactions and a notable increase in the complexity of the reported structures. This review covers key developments in the click chemistry field applied to dendrimer synthesis from 2010 to 2015. Even though this is an expert review, basic notions and references have been included to help newcomers to the field.
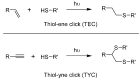
Keywords: Diels-Alder; Hüisgen cycloaddition; click; dendrimer; dendron; thiol-ene; thiol-yne.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Facile and Efficient Synthesis of Carbosiloxane Dendrimers via Orthogonal Click Chemistry Between Thiol and Ene.Macromol Rapid Commun. 2016 Feb;37(4):318-22. doi: 10.1002/marc.201500607. Epub 2015 Dec 16. Macromol Rapid Commun. 2016. PMID: 26676283
-
Click dendrimers and triazole-related aspects: catalysts, mechanism, synthesis, and functions. A bridge between dendritic architectures and nanomaterials.Acc Chem Res. 2012 Apr 17;45(4):630-40. doi: 10.1021/ar200235m. Epub 2011 Dec 8. Acc Chem Res. 2012. PMID: 22148925 Review.
-
Synthesis of cationic carbosilane dendrimers via click chemistry and their use as effective carriers for DNA transfection into cancerous cells.Mol Pharm. 2012 Mar 5;9(3):433-47. doi: 10.1021/mp200542j. Epub 2012 Jan 13. Mol Pharm. 2012. PMID: 22201256
-
Synthesis of Dense and Chiral Dendritic Polyols Using Glyconanosynthon Scaffolds.Molecules. 2016 Apr 4;21(4):448. doi: 10.3390/molecules21040448. Molecules. 2016. PMID: 27049377 Free PMC article.
-
Alginate modification via click chemistry for biomedical applications.Carbohydr Polym. 2021 Oct 15;270:118360. doi: 10.1016/j.carbpol.2021.118360. Epub 2021 Jun 19. Carbohydr Polym. 2021. PMID: 34364605 Review.
Cited by
-
Nanoparticle Therapeutics in Clinical Perspective: Classification, Marketed Products, and Regulatory Landscape.Small. 2025 Jul;21(29):e2502315. doi: 10.1002/smll.202502315. Epub 2025 Jun 2. Small. 2025. PMID: 40454890 Free PMC article. Review.
-
Assessment of TEMPO as a Thermally Activatable Base Generator and Its Use in Initiation of Thermally-Triggered Thiol-Michael Addition Polymerizations.Polym Chem. 2018 Aug 28;9(32):4294-4302. doi: 10.1039/C8PY00662H. Epub 2018 Jul 14. Polym Chem. 2018. PMID: 30740149 Free PMC article.
-
Breaking barriers: Smart vaccine platforms for cancer immunomodulation.Cancer Commun (Lond). 2025 May;45(5):529-571. doi: 10.1002/cac2.70002. Epub 2025 Feb 3. Cancer Commun (Lond). 2025. PMID: 39901621 Free PMC article. Review.
-
Development of Essential Oil Delivery Systems by 'Click Chemistry' Methods: Possible Ways to Manage Duchenne Muscular Dystrophy.Materials (Basel). 2023 Oct 2;16(19):6537. doi: 10.3390/ma16196537. Materials (Basel). 2023. PMID: 37834674 Free PMC article. Review.
-
The Novel Synthetic Antibiotic BDTL049 Based on a Dendritic System Induces Lipid Domain Formation while Escaping the Cell Envelope Stress Resistance Determinants.Pharmaceutics. 2023 Jan 16;15(1):297. doi: 10.3390/pharmaceutics15010297. Pharmaceutics. 2023. PMID: 36678925 Free PMC article.
References
-
- Wu P., Feldman A.K., Nugent A.K., Hawker C.J., Scheel A., Voit B., Pyun J., Fréchet J.M.J., Sharpless K.B., Fokin V.V. Efficiency and Fidelity in a Click-Chemistry Route to Triazole Dendrimers by the Copper(I)-Catalyzed Ligation of Azides and Alkynes. Angew. Chem. Int. Ed. 2004;43:3928–3932. doi: 10.1002/anie.200454078. - DOI - PubMed
-
- Gao C., Yan D. Hyperbranched polymers: From synthesis to applications. Prog. Polym. Sci. 2004;29:183–275. doi: 10.1016/j.progpolymsci.2003.12.002. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

