Palladium-catalyzed modification of unprotected nucleosides, nucleotides, and oligonucleotides
- PMID: 26007192
- PMCID: PMC6272472
- DOI: 10.3390/molecules20059419
Palladium-catalyzed modification of unprotected nucleosides, nucleotides, and oligonucleotides
Abstract
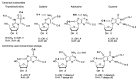
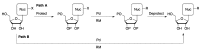
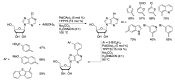
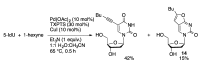
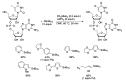
Synthetic modification of nucleoside structures provides access to molecules of interest as pharmaceuticals, biochemical probes, and models to study diseases. Covalent modification of the purine and pyrimidine bases is an important strategy for the synthesis of these adducts. Palladium-catalyzed cross-coupling is a powerful method to attach groups to the base heterocycles through the formation of new carbon-carbon and carbon-heteroatom bonds. In this review, approaches to palladium-catalyzed modification of unprotected nucleosides, nucleotides, and oligonucleotides are reviewed. Polar reaction media, such as water or polar aprotic solvents, allow reactions to be performed directly on the hydrophilic nucleosides and nucleotides without the need to use protecting groups. Homogeneous aqueous-phase coupling reactions catalyzed by palladium complexes of water-soluble ligands provide a general approach to the synthesis of modified nucleosides, nucleotides, and oligonucleotides.
Keywords: aqueous-phase catalysis; cross-coupling; nucleosides; nucleotides; oligonucleotides; palladium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
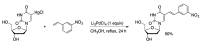
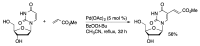




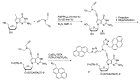
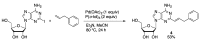
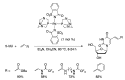
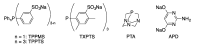
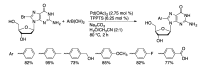
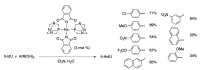
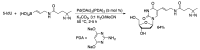
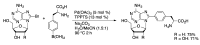


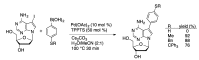
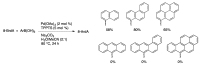
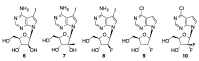
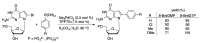
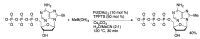
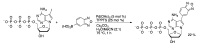
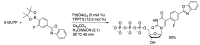
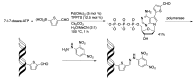
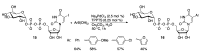
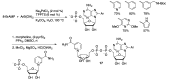

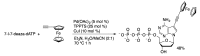
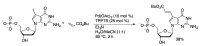

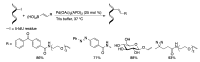
Similar articles
-
Covalent Modification of Nucleobases using Water-Soluble Palladium Catalysts.Chem Rec. 2022 Dec;22(12):e202200190. doi: 10.1002/tcr.202200190. Epub 2022 Sep 8. Chem Rec. 2022. PMID: 36074958 Review.
-
Water-Soluble Pd-Imidate Complexes as Versatile Catalysts for the Modification of Unprotected Halonucleosides.Chem Rec. 2022 Dec;22(12):e202200179. doi: 10.1002/tcr.202200179. Epub 2022 Sep 12. Chem Rec. 2022. PMID: 36094784 Review.
-
Palladium-catalyzed direct N-arylation of nucleosides, nucleotides, and oligonucleotides for efficient preparation of dG-N2 adducts with carcinogenic amino-/nitroarenes.J Org Chem. 2006 Jul 21;71(15):5599-606. doi: 10.1021/jo0605243. J Org Chem. 2006. PMID: 16839139
-
Discovery, Synthesis, and Scale-up of Efficient Palladium Catalysts Useful for the Modification of Nucleosides and Heteroarenes.Molecules. 2020 Apr 3;25(7):1645. doi: 10.3390/molecules25071645. Molecules. 2020. PMID: 32260100 Free PMC article. Review.
-
Synthesis of Nonnatural Oligonucleotides Made Exclusively of Alkynyl C-Nucleosides with Nonnatural Bases.Curr Protoc Nucleic Acid Chem. 2015 Jun 3;61:4.62.1-4.62.22. doi: 10.1002/0471142700.nc0462s61. Curr Protoc Nucleic Acid Chem. 2015. PMID: 26344228
Cited by
-
Tuning of Oxidation Potential of Ferrocene for Ratiometric Redox Labeling and Coding of Nucleotides and DNA.Chemistry. 2020 Jan 27;26(6):1286-1291. doi: 10.1002/chem.201904700. Epub 2020 Jan 9. Chemistry. 2020. PMID: 31725178 Free PMC article.
-
Modified Nucleotides as Substrates of Terminal Deoxynucleotidyl Transferase.Molecules. 2017 Apr 22;22(4):672. doi: 10.3390/molecules22040672. Molecules. 2017. PMID: 28441732 Free PMC article.
-
Modification of purine and pyrimidine nucleosides by direct C-H bond activation.Molecules. 2015 Mar 17;20(3):4874-901. doi: 10.3390/molecules20034874. Molecules. 2015. PMID: 25789821 Free PMC article. Review.
-
Tuning Cross-Coupling Approaches to C3 Modification of 3-Deazapurines.ACS Omega. 2017 Oct 31;2(10):7002-7015. doi: 10.1021/acsomega.7b01159. Epub 2017 Oct 20. ACS Omega. 2017. PMID: 30023537 Free PMC article.
-
Synthesis and Evaluation of Diguanosine Cap Analogs Modified at the C8-Position by Suzuki-Miyaura Cross-Coupling: Discovery of 7-Methylguanosine-Based Molecular Rotors.J Org Chem. 2023 Jun 2;88(11):6827-6846. doi: 10.1021/acs.joc.3c00126. Epub 2023 May 20. J Org Chem. 2023. PMID: 37209102 Free PMC article.
References
-
- Robak T. New Purine Nucleoside Analogs for Acute Lymphoblastic Leukemia. Clin. Cancer Drugs. 2014;1:2–10. doi: 10.2174/2212697X01999131126150545. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

