Clinical, Molecular, and Functional Characterization of CLCN1 Mutations in Three Families with Recessive Myotonia Congenita
- PMID: 26007199
- PMCID: PMC4534513
- DOI: 10.1007/s12017-015-8356-8
Clinical, Molecular, and Functional Characterization of CLCN1 Mutations in Three Families with Recessive Myotonia Congenita
Abstract
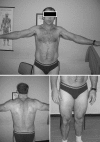
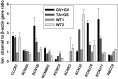
Myotonia congenita (MC) is an inherited muscle disease characterized by impaired muscle relaxation after contraction, resulting in muscle stiffness. Both recessive (Becker's disease) or dominant (Thomsen's disease) MC are caused by mutations in the CLCN1 gene encoding the voltage-dependent chloride ClC-1 channel, which is quite exclusively expressed in skeletal muscle. More than 200 CLCN1 mutations have been associated with MC. We provide herein a detailed clinical, molecular, and functional evaluation of four patients with recessive MC belonging to three different families. Four CLCN1 variants were identified, three of which have never been characterized. The c.244A>G (p.T82A) and c.1357C>T (p.R453W) variants were each associated in compound heterozygosity with c.568GG>TC (p.G190S), for which pathogenicity is already known. The new c.809G>T (p.G270V) variant was found in the homozygous state. Patch-clamp studies of ClC-1 mutants expressed in tsA201 cells confirmed the pathogenicity of p.G270V, which greatly shifts the voltage dependence of channel activation toward positive potentials. Conversely, the mechanisms by which p.T82A and p.R453W cause the disease remained elusive, as the mutated channels behave similarly to WT. The results also suggest that p.G190S does not exert dominant-negative effects on other mutated ClC-1 subunits. Moreover, we performed a RT-PCR quantification of selected ion channels transcripts in muscle biopsies of two patients. The results suggest gene expression alteration of sodium and potassium channel subunits in myotonic muscles; if confirmed, such analysis may pave the way toward a better understanding of disease phenotype and a possible identification of new therapeutic options.
Figures



Similar articles
-
Mutations in the human skeletal muscle chloride channel gene (CLCN1) associated with dominant and recessive myotonia congenita.Neurology. 1996 Oct;47(4):993-8. doi: 10.1212/wnl.47.4.993. Neurology. 1996. PMID: 8857733
-
ClC-1 mutations in myotonia congenita patients: insights into molecular gating mechanisms and genotype-phenotype correlation.J Physiol. 2015 Sep 15;593(18):4181-99. doi: 10.1113/JP270358. Epub 2015 Jul 14. J Physiol. 2015. PMID: 26096614 Free PMC article.
-
Becker's myotonia: novel mutations and clinical variability in patients born to consanguineous parents.Acta Neurol Belg. 2018 Dec;118(4):567-572. doi: 10.1007/s13760-018-0893-0. Epub 2018 Feb 26. Acta Neurol Belg. 2018. PMID: 29480456
-
Myotonia caused by mutations in the muscle chloride channel gene CLCN1.Hum Mutat. 2002 Apr;19(4):423-34. doi: 10.1002/humu.10063. Hum Mutat. 2002. PMID: 11933197 Review.
-
Clinical and molecular characteristics of myotonia congenita in China: Case series and a literature review.Channels (Austin). 2022 Dec;16(1):35-46. doi: 10.1080/19336950.2022.2041292. Channels (Austin). 2022. PMID: 35170402 Free PMC article. Review.
Cited by
-
Long-Term Safety and Usefulness of Mexiletine in a Large Cohort of Patients Affected by Non-dystrophic Myotonias.Front Neurol. 2020 May 20;11:300. doi: 10.3389/fneur.2020.00300. eCollection 2020. Front Neurol. 2020. PMID: 32655465 Free PMC article.
-
ClC-1 Chloride Channel: Inputs on the Structure-Function Relationship of Myotonia Congenita-Causing Mutations.Biomedicines. 2023 Sep 24;11(10):2622. doi: 10.3390/biomedicines11102622. Biomedicines. 2023. PMID: 37892996 Free PMC article. Review.
-
Dystrophia myotonica type 1 presenting with dysarthria: A case report and literature review.Exp Ther Med. 2017 Aug;14(2):1104-1108. doi: 10.3892/etm.2017.4579. Epub 2017 Jun 12. Exp Ther Med. 2017. PMID: 28810563 Free PMC article.
-
Impaired surface membrane insertion of homo- and heterodimeric human muscle chloride channels carrying amino-terminal myotonia-causing mutations.Sci Rep. 2015 Oct 27;5:15382. doi: 10.1038/srep15382. Sci Rep. 2015. PMID: 26502825 Free PMC article.
-
Therapeutic Approaches to Genetic Ion Channelopathies and Perspectives in Drug Discovery.Front Pharmacol. 2016 May 10;7:121. doi: 10.3389/fphar.2016.00121. eCollection 2016. Front Pharmacol. 2016. PMID: 27242528 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

