Perimenopause as a neurological transition state
- PMID: 26007613
- PMCID: PMC9934205
- DOI: 10.1038/nrendo.2015.82
Perimenopause as a neurological transition state
Abstract
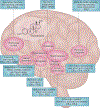
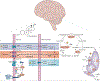
Perimenopause is a midlife transition state experienced by women that occurs in the context of a fully functioning neurological system and results in reproductive senescence. Although primarily viewed as a reproductive transition, the symptoms of perimenopause are largely neurological in nature. Neurological symptoms that emerge during perimenopause are indicative of disruption in multiple estrogen-regulated systems (including thermoregulation, sleep, circadian rhythms and sensory processing) and affect multiple domains of cognitive function. Estrogen is a master regulator that functions through a network of estrogen receptors to ensure that the brain effectively responds at rapid, intermediate and long timescales to regulate energy metabolism in the brain via coordinated signalling and transcriptional pathways. The estrogen receptor network becomes uncoupled from the bioenergetic system during the perimenopausal transition and, as a corollary, a hypometabolic state associated with neurological dysfunction can develop. For some women, this hypometabolic state might increase the risk of developing neurodegenerative diseases later in life. The perimenopausal transition might also represent a window of opportunity to prevent age-related neurological diseases. This Review considers the importance of neurological symptoms in perimenopause in the context of their relationship to the network of estrogen receptors that control metabolism in the brain.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


References
-
- Brinton RD in Brockelhurst’s Textbook of Geriatric Medicine and Gerontology (eds Fillit H, Rockwood K & Young J) 163–171 (Saunders, 2010).
-
- United States Census Bureau. World population by age and sex, [online], http://www.census.gov/population/international/data/worldpop/tool_popula... (2014).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

