A Highly Sensitive Porous Silicon (P-Si)-Based Human Kallikrein 2 (hK2) Immunoassay Platform toward Accurate Diagnosis of Prostate Cancer
- PMID: 26007739
- PMCID: PMC4481930
- DOI: 10.3390/s150511972
A Highly Sensitive Porous Silicon (P-Si)-Based Human Kallikrein 2 (hK2) Immunoassay Platform toward Accurate Diagnosis of Prostate Cancer
Abstract
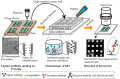
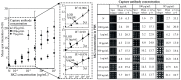
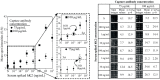
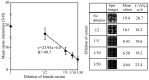
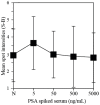
Levels of total human kallikrein 2 (hK2), a protein involved the pathology of prostate cancer (PCa), could be used as a biomarker to aid in the diagnosis of this disease. In this study, we report on a porous silicon antibody immunoassay platform for the detection of serum levels of total hK2. The surface of porous silicon has a 3-dimensional macro- and nanoporous structure, which offers a large binding capacity for capturing probe molecules. The tailored pore size of the porous silicon also allows efficient immobilization of antibodies by surface adsorption, and does not require chemical immobilization. Monoclonal hK2 capture antibody (6B7) was dispensed onto P-Si chip using a piezoelectric dispenser. In total 13 × 13 arrays (169 spots) were spotted on the chip with its single spot volume of 300 pL. For an optimization of capture antibody condition, we firstly performed an immunoassay of the P-Si microarray under a titration series of hK2 in pure buffer (PBS) at three different antibody densities (75, 100 and 145 µg/mL). The best performance of the microarray platform was seen at 100 µg/mL of the capture antibody concentration (LOD was 100 fg/mL). The platform then was subsequently evaluated for a titration series of serum-spiked hK2 samples. The developed platform utilizes only 15 µL of serum per test and the total assay time is about 3 h, including immobilization of the capture antibody. The detection limit of the hK2 assay was 100 fg/mL in PBS buffer and 1 pg/mL in serum with a dynamic range of 106 (10(-4) to 10(2) ng/mL).
Keywords: antibody microarrays; human kallikrein 2; porous silicon; prostate cancer.
Figures

Similar articles
-
Improved porous silicon microarray based prostate specific antigen immunoassay by optimized surface density of the capture antibody.Anal Chim Acta. 2013 Sep 24;796:108-14. doi: 10.1016/j.aca.2013.06.041. Epub 2013 Jul 3. Anal Chim Acta. 2013. PMID: 24016590 Free PMC article.
-
Macro-/nanoporous silicon as a support for high-performance protein microarrays.Anal Chem. 2003 Dec 15;75(24):6968-74. doi: 10.1021/ac034425q. Anal Chem. 2003. PMID: 14670060
-
The importance of human glandular kallikrein and its correlation with different prostate specific antigen serum forms in the detection of prostate carcinoma.Cancer. 1998 Dec 15;83(12):2540-7. Cancer. 1998. PMID: 9874461
-
Prostate-specific human kallikrein (hK2) as a novel marker for prostate cancer.Prostate Suppl. 1996;7:17-24. Prostate Suppl. 1996. PMID: 8950360 Review.
-
Screening for prostate cancer in 2008 II: the importance of molecular subforms of prostate-specific antigen and tissue kallikreins.Eur Urol. 2009 Mar;55(3):563-74. doi: 10.1016/j.eururo.2008.11.040. Epub 2008 Nov 29. Eur Urol. 2009. PMID: 19058905 Review.
Cited by
-
Prostate cancer markers: An update.Biomed Rep. 2016 Mar;4(3):263-268. doi: 10.3892/br.2016.586. Epub 2016 Jan 29. Biomed Rep. 2016. PMID: 26998261 Free PMC article.
-
Effect of miR-200c on proliferation, invasion and apoptosis of prostate cancer LNCaP cells.Oncol Lett. 2019 May;17(5):4299-4304. doi: 10.3892/ol.2019.10102. Epub 2019 Mar 4. Oncol Lett. 2019. PMID: 30944624 Free PMC article.
-
Nanomaterial-Modified Conducting Paper: Fabrication, Properties, and Emerging Biomedical Applications.Glob Chall. 2019 Sep 9;3(12):1900041. doi: 10.1002/gch2.201900041. eCollection 2019 Dec. Glob Chall. 2019. PMID: 31832235 Free PMC article. Review.
References
-
- Vickers A.J., Ulmert D., Serio A.M., Bjork T., Scardino P.T., Eastham J.A., Berglund G., Lilja H. The predictive value of prostate cancer biomarkers depends on age and time to diagnosis: Towards a biologically-based screening strategy. Int. J. Cancer. 2007;121:2212–2217. doi: 10.1002/ijc.22956. - DOI - PubMed
-
- Diamonds E.F., Yousef G.M. Human tissue kallikreins: A family of new cancer biomarkers. Clin. Chem. 2002;48:1198–1205. - PubMed
-
- Vickers A., Cronin A., Roobol M., Savage C., Peltola M., Pettersson K., Scardino P.T., Schroder F., Lilja H. Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: An independent replication. J. Clin. Oncol. 2010;28:2493–2498. doi: 10.1200/JCO.2009.24.1968. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

