Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes
- PMID: 26008129
- PMCID: PMC4877133
- DOI: 10.1038/nrgastro.2015.74
Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes
Abstract
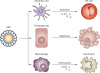
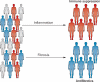
Biliary atresia is a severe cholangiopathy of early infancy that destroys extrahepatic bile ducts and disrupts bile flow. With a poorly defined disease pathogenesis, treatment consists of the surgical removal of duct remnants followed by hepatoportoenterostomy. Although this approach can improve the short-term outcome, the liver disease progresses to end-stage cirrhosis in most children. Further improvement in outcome will require a greater understanding of the mechanisms of biliary injury and fibrosis. Here, we review progress in the field, which has been fuelled by collaborative studies in larger patient cohorts and the development of cell culture and animal model systems to directly test hypotheses. Advances include the identification of phenotypic subgroups and stages of disease based on clinical, pathological and molecular features. Stronger evidence exists for viruses, toxins and gene sequence variations in the aetiology of biliary atresia, triggering a proinflammatory response that injures the duct epithelium and produces a rapidly progressive cholangiopathy. The immune response also activates the expression of type 2 cytokines that promote epithelial cell proliferation and extracellular matrix production by nonparenchymal cells. These advances provide insight into phenotype variability and might be relevant to the design of personalized trials to block progression of liver disease.
Figures

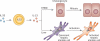
References
-
- Lee WS, Chai PF, Boey CM, Looi LM. Aetiology and outcome of neonatal cholestasis in Malaysia. Singapore Med. J. 2010;51:434–439. - PubMed
-
- Stormon MO, Dorney SF, Kamath KR, O’Loughlin EV, Gaskin KJ. The changing pattern of diagnosis of infantile cholestasis. J. Paediatr. Child Health. 2001;37:47–50. - PubMed
-
- Schreiber RA, Kleinman RE. Biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2002;35(Suppl. 1):S11–S16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

