A gold nanohole array based surface-enhanced Raman scattering biosensor for detection of silver(I) and mercury(II) in human saliva
- PMID: 26008641
- PMCID: PMC4476066
- DOI: 10.1039/c5nr02142a
A gold nanohole array based surface-enhanced Raman scattering biosensor for detection of silver(I) and mercury(II) in human saliva
Abstract
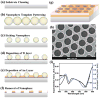
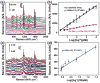
A surface-enhanced Raman scattering (SERS) biosensor has been developed by incorporating a gold nanohole array with a SERS probe (a gold nanostar@Raman-reporter@silica sandwich structure) into a single detection platform via DNA hybridization, which circumvents the nanoparticle aggregation and the inefficient Raman scattering issues. Strong plasmonic coupling between the Au nanostar and the Au nanohole array results in a large enhancement of the electromagnetic field, leading to amplification of the SERS signal. The SERS sensor has been used to detect Ag(I) and Hg(II) ions in human saliva because both the metal ions could be released from dental amalgam fillings. The developed SERS sensor can be adapted as a general detection platform for non-invasive measurements of a wide range of analytes such as metal ions, small molecules, DNA and proteins in body fluids.
Figures


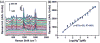

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

