Brain barriers: Crosstalk between complex tight junctions and adherens junctions
- PMID: 26008742
- PMCID: PMC4442813
- DOI: 10.1083/jcb.201412147
Brain barriers: Crosstalk between complex tight junctions and adherens junctions
Abstract
Unique intercellular junctional complexes between the central nervous system (CNS) microvascular endothelial cells and the choroid plexus epithelial cells form the endothelial blood-brain barrier (BBB) and the epithelial blood-cerebrospinal fluid barrier (BCSFB), respectively. These barriers inhibit paracellular diffusion, thereby protecting the CNS from fluctuations in the blood. Studies of brain barrier integrity during development, normal physiology, and disease have focused on BBB and BCSFB tight junctions but not the corresponding endothelial and epithelial adherens junctions. The crosstalk between adherens junctions and tight junctions in maintaining barrier integrity is an understudied area that may represent a promising target for influencing brain barrier function.
© 2015 Tietz and Engelhardt.
Figures
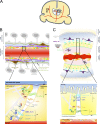

References
-
- Alvarez J.I., Saint-Laurent O., Godschalk A., Terouz S., Briels C., Larouche S., Bourbonnière L., Larochelle C., and Prat A.. 2015. Focal disturbances in the blood-brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol. Dis. 74:14–24. 10.1016/j.nbd.2014.09.016 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

