BLOC-2 targets recycling endosomal tubules to melanosomes for cargo delivery
- PMID: 26008744
- PMCID: PMC4442807
- DOI: 10.1083/jcb.201410026
BLOC-2 targets recycling endosomal tubules to melanosomes for cargo delivery
Abstract
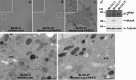
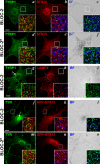
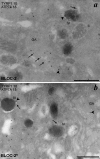
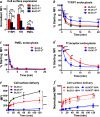
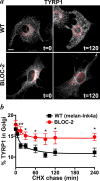
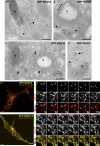
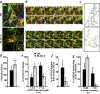
Hermansky-Pudlak syndrome (HPS) is a group of disorders characterized by the malformation of lysosome-related organelles, such as pigment cell melanosomes. Three of nine characterized HPS subtypes result from mutations in subunits of BLOC-2, a protein complex with no known molecular function. In this paper, we exploit melanocytes from mouse HPS models to place BLOC-2 within a cargo transport pathway from recycling endosomal domains to maturing melanosomes. In BLOC-2-deficient melanocytes, the melanosomal protein TYRP1 was largely depleted from pigment granules and underwent accelerated recycling from endosomes to the plasma membrane and to the Golgi. By live-cell imaging, recycling endosomal tubules of wild-type melanocytes made frequent and prolonged contacts with maturing melanosomes; in contrast, tubules from BLOC-2-deficient cells were shorter in length and made fewer, more transient contacts with melanosomes. These results support a model in which BLOC-2 functions to direct recycling endosomal tubular transport intermediates to maturing melanosomes and thereby promote cargo delivery and optimal pigmentation.
© 2015 Dennis et al.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

