DYRK1A controls the transition from proliferation to quiescence during lymphoid development by destabilizing Cyclin D3
- PMID: 26008897
- PMCID: PMC4451127
- DOI: 10.1084/jem.20150002
DYRK1A controls the transition from proliferation to quiescence during lymphoid development by destabilizing Cyclin D3
Abstract
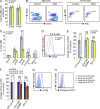
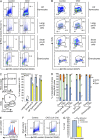
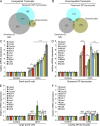
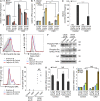
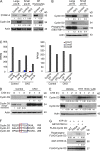
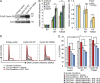
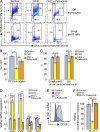
Pre-B and pre-T lymphocytes must orchestrate a transition from a highly proliferative state to a quiescent one during development. Cyclin D3 is essential for these cells' proliferation, but little is known about its posttranslational regulation at this stage. Here, we show that the dual specificity tyrosine-regulated kinase 1A (DYRK1A) restrains Cyclin D3 protein levels by phosphorylating T283 to induce its degradation. Loss of DYRK1A activity, via genetic inactivation or pharmacologic inhibition in mice, caused accumulation of Cyclin D3 protein, incomplete repression of E2F-mediated gene transcription, and failure to properly couple cell cycle exit with differentiation. Expression of a nonphosphorylatable Cyclin D3 T283A mutant recapitulated these defects, whereas inhibition of Cyclin D:CDK4/6 mitigated the effects of DYRK1A inhibition or loss. These data uncover a previously unknown role for DYRK1A in lymphopoiesis, and demonstrate how Cyclin D3 protein stability is negatively regulated during exit from the proliferative phases of B and T cell development.
© 2015 Thompson et al.
Figures



Similar articles
-
Dyrk1a regulates the cardiomyocyte cell cycle via D-cyclin-dependent Rb/E2f-signalling.Cardiovasc Res. 2016 Jun 1;110(3):381-94. doi: 10.1093/cvr/cvw074. Epub 2016 Apr 7. Cardiovasc Res. 2016. PMID: 27056896
-
Lymphocyte lineage-specific and developmental stage specific mechanisms suppress cyclin D3 expression in response to DNA double strand breaks.Cell Cycle. 2016 Nov;15(21):2882-2894. doi: 10.1080/15384101.2016.1198861. Epub 2016 Jun 21. Cell Cycle. 2016. PMID: 27327568 Free PMC article.
-
Expression and activity of the retinoblastoma protein (pRB)-family proteins, p107 and p130, during L6 myoblast differentiation.Cell Growth Differ. 1995 Oct;6(10):1287-98. Cell Growth Differ. 1995. PMID: 8845306
-
Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling.Nat Rev Immunol. 2014 Feb;14(2):69-80. doi: 10.1038/nri3570. Epub 2013 Dec 31. Nat Rev Immunol. 2014. PMID: 24378843 Free PMC article. Review.
-
No T without D3: a critical role for cyclin D3 in normal and malignant precursor T cells.Cancer Cell. 2003 Dec;4(6):417-8. doi: 10.1016/s1535-6108(03)00305-2. Cancer Cell. 2003. PMID: 14706330 Review.
Cited by
-
B cell class switch recombination is regulated by DYRK1A through MSH6 phosphorylation.Nat Commun. 2023 Mar 16;14(1):1462. doi: 10.1038/s41467-023-37205-5. Nat Commun. 2023. PMID: 36927854 Free PMC article.
-
iAMPlified gene expression offers new insights in B cell precursor leukemia subtype.Leuk Lymphoma. 2020 Mar;61(3):501-503. doi: 10.1080/10428194.2019.1695055. Epub 2020 Feb 3. Leuk Lymphoma. 2020. PMID: 32008406 Free PMC article. No abstract available.
-
SH2B3 inactivation through CN-LOH 12q is uniquely associated with B-cell precursor ALL with iAMP21 or other chromosome 21 gain.Leukemia. 2019 Aug;33(8):1881-1894. doi: 10.1038/s41375-019-0412-1. Epub 2019 Feb 28. Leukemia. 2019. PMID: 30816328 Free PMC article.
-
Cyclin-dependent kinase 1 (CDK1) and CDK2 have opposing roles in regulating interactions of splicing factor 3B1 with chromatin.J Biol Chem. 2018 Jun 29;293(26):10220-10234. doi: 10.1074/jbc.RA118.001654. Epub 2018 May 15. J Biol Chem. 2018. PMID: 29764937 Free PMC article.
-
The chromosome 21 kinase DYRK1A: emerging roles in cancer biology and potential as a therapeutic target.Oncogene. 2022 Apr;41(14):2003-2011. doi: 10.1038/s41388-022-02245-6. Epub 2022 Feb 26. Oncogene. 2022. PMID: 35220406 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

