Lemur Tyrosine Kinase 2, a novel target in prostate cancer therapy
- PMID: 26008968
- PMCID: PMC4546463
- DOI: 10.18632/oncotarget.3899
Lemur Tyrosine Kinase 2, a novel target in prostate cancer therapy
Abstract
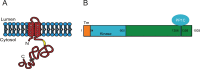
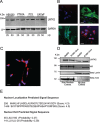
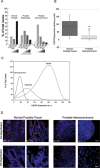
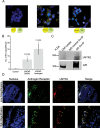
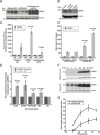
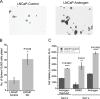
Progression from early forms of prostate cancer to castration-resistant disease is associated with an increase in signal transduction activity. The majority of castration-resistance cancers persist in the expression of the androgen receptor (AR), as well as androgen-dependent genes. The AR is regulated not only by it associated steroid hormone, but also by manifold regulatory and signaling molecules, including several kinases. We undertook evaluation of the role of Lemur Tyrosine Kinase 2 (LMTK2) in modulating AR activity, as several Genome Wide Association Studies (GWAS) have shown a marked association of LMTK2 activity with the development of prostate cancer. We confirm that not only is LMTK2 mRNA reduced in prostate cancer tissue, but also LMTK2 protein levels are markedly diminished. Knockdown of LMTK2 protein in prostate cell lines greatly increased the transcription of androgen-responsive genes. In addition, LMTK2 knockdown led to an increase in prostate cancer stem cell populations in LNCaP cells, indicative of increased tumorogenicity. Using multiple approaches, we also demonstrate that LMTK2 interacts with the AR, thus putting LMTK2 as a component of a signaling complex modulating AR activity. Our finding that LMTK2 is a negative regulator of AR activity defines a novel cellular pathway for activation of AR-responsive genes in castrate resistant-prostate cancer. Moreover, pharmacologic manipulation of LMTK2 activity will provide a novel therapeutic target for more effective treatments for patients with castrate-resistant prostate cancer.
Keywords: LMTK2; androgen receptor; castrate resistant prostate cancer; kinases; prostate cancer.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures
Similar articles
-
Nucleoporin 62 and Ca(2+)/calmodulin dependent kinase kinase 2 regulate androgen receptor activity in castrate resistant prostate cancer cells.Prostate. 2016 Feb 15;76(3):294-306. doi: 10.1002/pros.23121. Epub 2015 Nov 10. Prostate. 2016. PMID: 26552607
-
Identification of novel genes that regulate androgen receptor signaling and growth of androgen-deprived prostate cancer cells.Oncotarget. 2015 May 30;6(15):13088-104. doi: 10.18632/oncotarget.3743. Oncotarget. 2015. PMID: 26036626 Free PMC article.
-
Identification of kinases regulating prostate cancer cell growth using an RNAi phenotypic screen.PLoS One. 2012;7(6):e38950. doi: 10.1371/journal.pone.0038950. Epub 2012 Jun 27. PLoS One. 2012. PMID: 22761715 Free PMC article.
-
Androgen receptors in hormone-dependent and castration-resistant prostate cancer.Pharmacol Ther. 2013 Dec;140(3):223-38. doi: 10.1016/j.pharmthera.2013.07.003. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859952 Review.
-
Strategies for targeting the androgen receptor axis in prostate cancer.Drug Discov Today. 2014 Sep;19(9):1493-7. doi: 10.1016/j.drudis.2014.07.008. Epub 2014 Aug 10. Drug Discov Today. 2014. PMID: 25107669 Review.
Cited by
-
Kinase modulation of androgen receptor signaling: implications for prostate cancer.Cancer Cell Microenviron. 2015;2(4):e123. doi: 10.14800/ccm.1023. Epub 2015 Nov 19. Cancer Cell Microenviron. 2015. PMID: 28580371 Free PMC article.
-
LMTK3 confers chemo-resistance in breast cancer.Oncogene. 2018 Jun;37(23):3113-3130. doi: 10.1038/s41388-018-0197-0. Epub 2018 Mar 15. Oncogene. 2018. PMID: 29540829 Free PMC article.
-
Whole-exome sequencing of Nigerian benign prostatic hyperplasia reveals increased alterations in apoptotic pathways.Prostate. 2024 Apr;84(5):460-472. doi: 10.1002/pros.24662. Epub 2024 Jan 8. Prostate. 2024. PMID: 38192023 Free PMC article.
-
Lemur Tyrosine Kinases and Prostate Cancer: A Literature Review.Int J Mol Sci. 2021 May 21;22(11):5453. doi: 10.3390/ijms22115453. Int J Mol Sci. 2021. PMID: 34064250 Free PMC article. Review.
-
Unraveling the Function of Lemur Tyrosine Kinase 2 Network.Front Pharmacol. 2019 Jan 29;10:24. doi: 10.3389/fphar.2019.00024. eCollection 2019. Front Pharmacol. 2019. PMID: 30761001 Free PMC article. Review.
References
-
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annual review of biochemistry. 1994;63:451–486. - PubMed
-
- Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–326. - PubMed
-
- Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13498–13503. - PMC - PubMed
-
- De Marzo AM, Nelson WG, Meeker AK, Coffey DS. Stem cell features of benign and malignant prostate epithelial cells. The Journal of urology. 1998;160:2381–2392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

