Essential role for cyclic-AMP responsive element binding protein 1 (CREB) in the survival of acute lymphoblastic leukemia
- PMID: 26008971
- PMCID: PMC4558129
- DOI: 10.18632/oncotarget.3911
Essential role for cyclic-AMP responsive element binding protein 1 (CREB) in the survival of acute lymphoblastic leukemia
Abstract
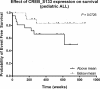
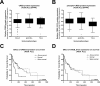
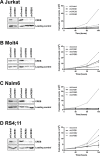
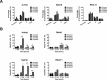
Acute lymphoblastic leukemia (ALL) relapse remains a leading cause of cancer related death in children, therefore, new therapeutic options are needed. Recently, we showed that a peptide derived from Cyclic-AMP Responsive Element Binding Protein (CREB) was highly phosphorylated in pediatric leukemias. In this study, we determined CREB phosphorylation and mRNA levels showing that CREB expression was significantly higher in ALL compared to normal bone marrow (phosphorylation: P < 0.0001, mRNA: P = 0.004). High CREB and phospho-CREB expression was correlated with a lower median overall survival in a cohort of 140 adult ALL patients. ShRNA mediated knockdown of CREB in ALL cell lines blocked leukemic cell growth by inducing cell cycle arrest and apoptosis. Gene expression array analysis showed downregulation of CREB target genes regulating cell proliferation and glucose metabolism and upregulation of apoptosis inducing genes. Similar to CREB knockdown, the CREB inhibitor KG-501 decreased leukemic cell viability and induced apoptosis in ALL cell lines, as well as primary T-ALL samples, with cases showing high phospho-CREB levels being more sensitive than those with lower phospho-CREB levels. Together, these in vitro findings support an important role for CREB in the survival of ALL cells and identify this transcription factor as a potential target for treatment.
Keywords: CREB; acute lymphoblastic leukemia; cyclic-AMP responsive element binding protein; targeted therapy.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures


References
-
- Mullighan CG. The molecular genetic makeup of acute lymphoblastic leukemia. Hematology Am.Soc.Hematol.Educ.Program. 2012;2012:389–396. - PubMed
-
- Roberts KG, Mullighan CG. How new advances in genetic analysis are influencing the understanding and treatment of childhood acute leukemia. Curr.Opin.Pediatr. 2011;23:34–40. - PubMed
-
- Ter Elst A, Diks SH, Kampen KR, Hoogerbrugge PM, Ruijtenbeek R, Boender PJ, Sikkema AH, Scherpen FJ, Kamps WA, Peppelenbosch MP, de Bont ES. Identification of new possible targets for leukemia treatment by kinase activity profiling. Leuk.Lymphoma. 2011;52:122–130. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

