Acetylation at lysine 71 inactivates superoxide dismutase 1 and sensitizes cancer cells to genotoxic agents
- PMID: 26008972
- PMCID: PMC4653027
- DOI: 10.18632/oncotarget.3987
Acetylation at lysine 71 inactivates superoxide dismutase 1 and sensitizes cancer cells to genotoxic agents
Abstract
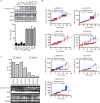
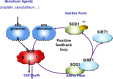
Cancer cells are characterized by a high dependency on antioxidant enzymes to cope with the elevated rates of reactive oxygen species (ROS). Impairing antioxidant capacity in cancer cells disturbs the ROS homeostasis and exposes cancer cells to massive oxidative stress. In this study, we have discovered that superoxide dismutase 1 (SOD1), a major player in maintaining the cellular redox status, was acetylated at lysine 71. This acetylation, which was primarily deacetylated by Sirtuin 1 (SIRT1), suppressed the enzymatic activity of SOD1 via disrupting its association with copper chaperone for SOD1 (CCS). More importantly, genotoxic agents, such as camptothecin (CPT), induced SOD1 acetylation by disrupting its binding with SIRT1. CPT-induced SOD1 acetylation was stimulated by its provoked ROS, suggesting a positive feedback loop, in which ROS per se impairs the antioxidative defence of cancer cells and reinforces oxidative stress stimulated by anticancer agents. The intrinsic abundance of SOD1 acetylation varied among cancer cells, and high level of SOD1 acetylation was correlated with elevated sensitivity to CPT. Together, our findings gained mechanistic insights into how cytotoxic agents fine tune the intracellular ROS homeostasis to strengthen their anticancer effects, and suggested SOD1 acetylation as a candidate biomarker for predicting response to CPT-based chemotherapy.
Keywords: SIRT1; acetylation; camptothecin; oxidative stress; superoxide dismutase.
Conflict of interest statement
The authors declared no conflict of interest.
Figures




Similar articles
-
Chronic Ethanol Metabolism Inhibits Hepatic Mitochondrial Superoxide Dismutase via Lysine Acetylation.Alcohol Clin Exp Res. 2017 Oct;41(10):1705-1714. doi: 10.1111/acer.13473. Epub 2017 Sep 14. Alcohol Clin Exp Res. 2017. PMID: 28804911 Free PMC article.
-
Sirtuin1-regulated lysine acetylation of p66Shc governs diabetes-induced vascular oxidative stress and endothelial dysfunction.Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):1714-1719. doi: 10.1073/pnas.1614112114. Epub 2017 Jan 30. Proc Natl Acad Sci U S A. 2017. PMID: 28137876 Free PMC article.
-
Sirtuin 1 attenuates oxidative stress via upregulation of superoxide dismutase 2 and catalase in astrocytes.J Neuroimmunol. 2014 Apr 15;269(1-2):38-43. doi: 10.1016/j.jneuroim.2014.02.001. Epub 2014 Feb 12. J Neuroimmunol. 2014. PMID: 24565075
-
Manganese Superoxide Dismutase Acetylation and Dysregulation, Due to Loss of SIRT3 Activity, Promote a Luminal B-Like Breast Carcinogenic-Permissive Phenotype.Antioxid Redox Signal. 2016 Aug 20;25(6):326-36. doi: 10.1089/ars.2016.6641. Epub 2016 Apr 15. Antioxid Redox Signal. 2016. PMID: 26935174 Free PMC article. Review.
-
Acetylation of MnSOD directs enzymatic activity responding to cellular nutrient status or oxidative stress.Aging (Albany NY). 2011 Feb;3(2):102-7. doi: 10.18632/aging.100291. Aging (Albany NY). 2011. PMID: 21386137 Free PMC article. Review.
Cited by
-
Dynamic acylome reveals metabolite driven modifications in Syntrophomonas wolfei.Front Microbiol. 2022 Nov 7;13:1018220. doi: 10.3389/fmicb.2022.1018220. eCollection 2022. Front Microbiol. 2022. PMID: 36419437 Free PMC article.
-
Parkinson-like wild-type superoxide dismutase 1 pathology induces nigral dopamine neuron degeneration in a novel murine model.Acta Neuropathol. 2025 Mar 5;149(1):22. doi: 10.1007/s00401-025-02859-6. Acta Neuropathol. 2025. PMID: 40042537 Free PMC article.
-
Chromosomal instability drives convergent and divergent evolution toward advantageous inherited traits in mammalian CHO bioproduction lineages.iScience. 2022 Mar 14;25(4):104074. doi: 10.1016/j.isci.2022.104074. eCollection 2022 Apr 15. iScience. 2022. PMID: 35355517 Free PMC article.
-
Acylation of Superoxide Dismutase 1 (SOD1) at K122 Governs SOD1-Mediated Inhibition of Mitochondrial Respiration.Mol Cell Biol. 2017 Sep 26;37(20):e00354-17. doi: 10.1128/MCB.00354-17. Print 2017 Oct 15. Mol Cell Biol. 2017. PMID: 28739857 Free PMC article.
-
Mechanisms of SOD1 regulation by post-translational modifications.Redox Biol. 2019 Sep;26:101270. doi: 10.1016/j.redox.2019.101270. Epub 2019 Jul 6. Redox Biol. 2019. PMID: 31344643 Free PMC article. Review.
References
-
- D'Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. - PubMed
-
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature reviews Drug discovery. 2009;8:579–591. - PubMed
-
- Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

