Piezo1 ion channel pore properties are dictated by C-terminal region
- PMID: 26008989
- PMCID: PMC4445471
- DOI: 10.1038/ncomms8223
Piezo1 ion channel pore properties are dictated by C-terminal region
Abstract
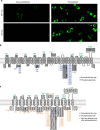
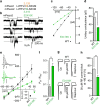
Piezo1 and Piezo2 encode mechanically activated cation channels that function as mechanotransducers involved in vascular system development and touch sensing, respectively. Structural features of Piezos remain unknown. Mouse Piezo1 is bioinformatically predicted to have 30-40 transmembrane (TM) domains. Here, we find that nine of the putative inter-transmembrane regions are accessible from the extracellular side. We use chimeras between mPiezo1 and dPiezo to show that ion-permeation properties are conferred by C-terminal region. We further identify a glutamate residue within a conserved region adjacent to the last two putative TM domains of the protein, that when mutated, affects unitary conductance and ion selectivity, and modulates pore block. We propose that this amino acid is either in the pore or closely associates with the pore. Our results describe important structural motifs of this channel family and lay the groundwork for a mechanistic understanding of how Piezos are mechanically gated and conduct ions.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

