Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer
- PMID: 26009480
- PMCID: PMC5528785
- DOI: 10.1016/j.molonc.2015.05.001
Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer
Abstract
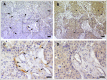
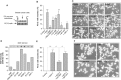
Infiltration of the tumor microenvironment by nerve fibers is an understudied aspect of breast carcinogenesis. In this study, the presence of nerve fibers was investigated in a cohort of 369 primary breast cancers (ductal carcinomas in situ, invasive ductal and lobular carcinomas) by immunohistochemistry for the neuronal marker PGP9.5. Isolated nerve fibers (axons) were detected in 28% of invasive ductal carcinomas as compared to only 12% of invasive lobular carcinomas and 8% of ductal carcinomas in situ (p = 0.0003). In invasive breast cancers, the presence of nerve fibers was observed in 15% of lymph node negative tumors and 28% of lymph node positive tumors (p = 0.0031), indicating a relationship with the metastatic potential. In addition, there was an association between the presence of nerve fibers and the expression of nerve growth factor (NGF) in cancer cells (p = 0.0001). In vitro, breast cancer cells were able to induce neurite outgrowth in PC12 cells, and this neurotrophic activity was partially inhibited by anti-NGF blocking antibodies. In conclusion, infiltration by nerve fibers is a feature of the tumor microenvironment that is associated with aggressiveness and involves NGF production by cancer cells. The potential participation of nerve fibers in breast cancer progression needs to be further considered.
Keywords: Breast cancer; Nerve fibers (axons); Nerve growth factor; Tumor microenvironment.
Copyright © 2015 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Adriaenssens, E. , Vanhecke, E. , Saule, P. , Mougel, A. , Page, A. , Romon, R. , Nurcombe, V. , Le Bourhis, X. , Hondermarck, H. , 2008. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 68, 346–351. - PubMed
-
- Bapat, A.A. , Hostetter, G. , Von Hoff, D.D. , Han, H. , 2011. Perineural invasion and associated pain in pancreatic cancer. Nat. Rev. Cancer. 11, 695–707. - PubMed
-
- Barron, T.I. , Connolly, R.M. , Sharp, L. , Bennett, K. , Visvanathan, K. , 2011. Beta blockers and breast cancer mortality: a population- based study. J. Clin. Oncol. 29, 2635–2644. - PubMed
-
- Bloom, A.P. , Jimenez-Andrade, J.M. , Taylor, R.N. , Castaneda-Corral, G. , Kaczmarska, M.J. , Freeman, K.T. , Coughlin, K.A. , Ghilardi, J.R. , Kuskowski, M.A. , Mantyh, P.W. , 2011. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J. Pain. 12, 698–711. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

