Investigation of potential artefactual changes in measurements of impedance changes during evoked activity: implications to electrical impedance tomography of brain function
- PMID: 26009486
- PMCID: PMC5390955
- DOI: 10.1088/0967-3334/36/6/1245
Investigation of potential artefactual changes in measurements of impedance changes during evoked activity: implications to electrical impedance tomography of brain function
Abstract
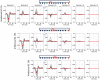
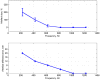
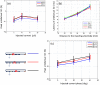
Electrical impedance tomography (EIT) could provide images of fast neural activity in the adult human brain with a resolution of 1 ms and 1 mm by imaging impedance changes which occur as ion channels open during neuronal depolarization. The largest changes occur at dc and decrease rapidly over 100 Hz. Evoked potentials occur in this bandwidth and may cause artefactual apparent impedance changes if altered by the impedance measuring current. These were characterized during the compound action potential in the walking leg nerves of Cancer pagurus, placed on Ag/AgCl hook electrodes, to identify how to avoid artefactual changes during brain EIT. Artefact-free impedance changes (δZ) decreased with frequency from -0.045 ± 0.01% at 225 Hz to -0.02 ± 0.01% at 1025 Hz (mean ± 1 SD, n = 24 in 12 nerves) which matched changes predicted by a finite element model. Artefactual δZ reached c.300% and 50% of the genuine membrane impedance change at 225 Hz and 600 Hz respectively but decreased with frequency of the applied current and was negligible above 1 kHz. The proportional amplitude (δZ (%)) of the artefact did not vary significantly with the amplitude of injected current of 5-20 µA pp. but decreased significantly from -0.09 ± 0.024 to -0.03 ± 0.023% with phase of 0 to 45°. For fast neural EIT of evoked activity in the brain, artefacts may arise with applied current of >10 µA. Independence of δZ with respect to phase but not the amplitude of applied current controls for them; they can be minimized by randomizing the phase of the applied measuring current and excluded by recording at >1 kHz.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
