The couplonopathies: A comparative approach to a class of diseases of skeletal and cardiac muscle
- PMID: 26009541
- PMCID: PMC4442791
- DOI: 10.1085/jgp.201411321
The couplonopathies: A comparative approach to a class of diseases of skeletal and cardiac muscle
Abstract
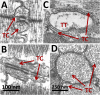
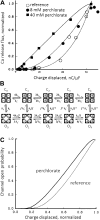
A novel category of diseases of striated muscle is proposed, the couplonopathies, as those that affect components of the couplon and thereby alter its operation. Couplons are the functional units of intracellular calcium release in excitation-contraction coupling. They comprise dihydropyridine receptors, ryanodine receptors (Ca2+ release channels), and a growing list of ancillary proteins whose alteration may lead to disease. Within a generally similar plan, the couplons of skeletal and cardiac muscle show, in a few places, marked structural divergence associated with critical differences in the mechanisms whereby they fulfill their signaling role. Most important among these are the presence of a mechanical or allosteric communication between voltage sensors and Ca2+ release channels, exclusive to the skeletal couplon, and the smaller capacity of the Ca stores in cardiac muscle, which results in greater swings of store concentration during physiological function. Consideration of these structural and functional differences affords insights into the pathogenesis of several couplonopathies. The exclusive mechanical connection of the skeletal couplon explains differences in pathogenesis between malignant hyperthermia (MH) and catecholaminergic polymorphic ventricular tachycardia (CPVT), conditions most commonly caused by mutations in homologous regions of the skeletal and cardiac Ca(2+) release channels. Based on mechanistic considerations applicable to both couplons, we identify the plasmalemma as a site of secondary modifications, typically an increase in store-operated calcium entry, that are relevant in MH pathogenesis. Similar considerations help explain the different consequences that mutations in triadin and calsequestrin have in these two tissues. As more information is gathered on the composition of cardiac and skeletal couplons, this comparative and mechanistic approach to couplonopathies should be useful to understand pathogenesis, clarify diagnosis, and propose tissue-specific drug development.
© 2015 Ríos et al.
Figures

Similar articles
-
Calsequestrin and the calcium release channel of skeletal and cardiac muscle.Prog Biophys Mol Biol. 2004 May;85(1):33-69. doi: 10.1016/j.pbiomolbio.2003.07.001. Prog Biophys Mol Biol. 2004. PMID: 15050380 Review.
-
Control of muscle ryanodine receptor calcium release channels by proteins in the sarcoplasmic reticulum lumen.Clin Exp Pharmacol Physiol. 2009 Mar;36(3):340-5. doi: 10.1111/j.1440-1681.2008.05094.x. Clin Exp Pharmacol Physiol. 2009. PMID: 19278523 Review.
-
The role of ion-regulatory membrane proteins of excitation-contraction coupling and relaxation in inherited muscle diseases.Front Biosci. 2001 Jan 1;6:D65-74. doi: 10.2741/froemmin. Front Biosci. 2001. PMID: 11145921 Review.
-
Junctin and triadin each activate skeletal ryanodine receptors but junctin alone mediates functional interactions with calsequestrin.Int J Biochem Cell Biol. 2009 Nov;41(11):2214-24. doi: 10.1016/j.biocel.2009.04.017. Epub 2009 May 4. Int J Biochem Cell Biol. 2009. PMID: 19398037 Free PMC article.
-
Ca(2+) signaling in striated muscle: the elusive roles of triadin, junctin, and calsequestrin.Eur Biophys J. 2009 Dec;39(1):27-36. doi: 10.1007/s00249-009-0449-6. Epub 2009 May 12. Eur Biophys J. 2009. PMID: 19434403 Review.
Cited by
-
Elevated Ca2+ at the triad junction underlies dysregulation of Ca2+ signaling in dysferlin-null skeletal muscle.Front Physiol. 2022 Nov 3;13:1032447. doi: 10.3389/fphys.2022.1032447. eCollection 2022. Front Physiol. 2022. PMID: 36406982 Free PMC article.
-
A 3D diffusional-compartmental model of the calcium dynamics in cytosol, sarcoplasmic reticulum and mitochondria of murine skeletal muscle fibers.PLoS One. 2018 Jul 26;13(7):e0201050. doi: 10.1371/journal.pone.0201050. eCollection 2018. PLoS One. 2018. PMID: 30048500 Free PMC article.
-
Stem Cells Associated with Adult Skeletal Muscle Can Form Beating Cardiac Tissue In Vitro in Response to Media Containing Heparin, Dexamethasone, Growth Factors and Hydrogen Peroxide.Int J Mol Sci. 2025 Mar 17;26(6):2683. doi: 10.3390/ijms26062683. Int J Mol Sci. 2025. PMID: 40141327 Free PMC article.
-
Functional impact of an oculopharyngeal muscular dystrophy mutation in PABPN1.J Physiol. 2017 Jul 1;595(13):4167-4187. doi: 10.1113/JP273948. Epub 2017 Apr 25. J Physiol. 2017. PMID: 28303574 Free PMC article.
-
The Orai1 inhibitor BTP2 has multiple effects on Ca2+ handling in skeletal muscle.J Gen Physiol. 2021 Jan 4;153(1):e202012747. doi: 10.1085/jgp.202012747. J Gen Physiol. 2021. PMID: 33316029 Free PMC article.
References
-
- Anderson A.A., Treves S., Biral D., Betto R., Sandonà D., Ronjat M., and Zorzato F.. 2003. The novel skeletal muscle sarcoplasmic reticulum JP-45 protein. Molecular cloning, tissue distribution, developmental expression, and interaction with α1.1 subunit of the voltage-gated calcium channel. J. Biol. Chem. 278:39987–39992. 10.1074/jbc.M305016200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

