Beyond non-integer Hill coefficients: A novel approach to analyzing binding data, applied to Na+-driven transporters
- PMID: 26009546
- PMCID: PMC4442788
- DOI: 10.1085/jgp.201511365
Beyond non-integer Hill coefficients: A novel approach to analyzing binding data, applied to Na+-driven transporters
Abstract
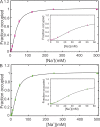
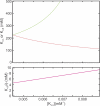
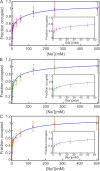
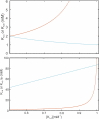
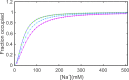
Prokaryotic and eukaryotic Na(+)-driven transporters couple the movement of one or more Na(+) ions down their electrochemical gradient to the active transport of a variety of solutes. When more than one Na(+) is involved, Na(+)-binding data are usually analyzed using the Hill equation with a non-integer exponent n. The results of this analysis are an overall Kd-like constant equal to the concentration of ligand that produces half saturation and n, a measure of cooperativity. This information is usually insufficient to provide the basis for mechanistic models. In the case of transport using two Na(+) ions, an n < 2 indicates that molecules with only one of the two sites occupied are present at low saturation. Here, we propose a new way of analyzing Na(+)-binding data for the case of two Na(+) ions that, by taking into account binding to individual sites, provides far more information than can be obtained by using the Hill equation with a non-integer coefficient: it yields pairs of possible values for the Na(+) affinities of the individual sites that can only vary within narrowly bounded ranges. To illustrate the advantages of the method, we present experimental scintillation proximity assay (SPA) data on binding of Na(+) to the Na(+)/I(-) symporter (NIS). SPA is a method widely used to study the binding of Na(+) to Na(+)-driven transporters. NIS is the key plasma membrane protein that mediates active I(-) transport in the thyroid gland, the first step in the biosynthesis of the thyroid hormones, of which iodine is an essential constituent. NIS activity is electrogenic, with a 2:1 Na(+)/I(-) transport stoichiometry. The formalism proposed here is general and can be used to analyze data on other proteins with two binding sites for the same substrate.
© 2015 Ravera et al.
Figures
Similar articles
-
Na+ coordination at the Na2 site of the Na+/I- symporter.Proc Natl Acad Sci U S A. 2016 Sep 13;113(37):E5379-88. doi: 10.1073/pnas.1607231113. Epub 2016 Aug 25. Proc Natl Acad Sci U S A. 2016. PMID: 27562170 Free PMC article.
-
The Hill analysis and co-ion-driven transporter kinetics.J Gen Physiol. 2015 Jun;145(6):565-74. doi: 10.1085/jgp.201411332. J Gen Physiol. 2015. PMID: 26009547 Free PMC article. Review.
-
Physiological sodium concentrations enhance the iodide affinity of the Na+/I- symporter.Nat Commun. 2014 Jun 3;5:3948. doi: 10.1038/ncomms4948. Nat Commun. 2014. PMID: 24888603 Free PMC article.
-
The Na+/I symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate.Proc Natl Acad Sci U S A. 2007 Dec 18;104(51):20250-5. doi: 10.1073/pnas.0707207104. Epub 2007 Dec 11. Proc Natl Acad Sci U S A. 2007. PMID: 18077370 Free PMC article.
-
Mechanistic aspects of sodium-binding sites in LeuT-like fold symporters.Biol Chem. 2013 May;394(5):641-8. doi: 10.1515/hsz-2012-0336. Biol Chem. 2013. PMID: 23362203 Review.
Cited by
-
A Carboxy-Terminal Monoleucine-Based Motif Participates in the Basolateral Targeting of the Na+/I- Symporter.Endocrinology. 2019 Jan 1;160(1):156-168. doi: 10.1210/en.2018-00603. Endocrinology. 2019. PMID: 30496374 Free PMC article.
-
Comparative analysis of the metal-dependent structural and functional properties of mouse and human SMP30.PLoS One. 2019 Jun 20;14(6):e0218629. doi: 10.1371/journal.pone.0218629. eCollection 2019. PLoS One. 2019. PMID: 31220150 Free PMC article.
-
Mapping of Ion and Substrate Binding Sites in Human Sodium Iodide Symporter (hNIS).J Chem Inf Model. 2020 Mar 23;60(3):1652-1665. doi: 10.1021/acs.jcim.9b01114. Epub 2020 Mar 12. J Chem Inf Model. 2020. PMID: 32134653 Free PMC article.
-
The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications.Annu Rev Physiol. 2017 Feb 10;79:261-289. doi: 10.1146/annurev-physiol-022516-034125. Annu Rev Physiol. 2017. PMID: 28192058 Free PMC article. Review.
-
Elevator-type mechanisms of membrane transport.Biochem Soc Trans. 2020 Jun 30;48(3):1227-1241. doi: 10.1042/BST20200290. Biochem Soc Trans. 2020. PMID: 32369548 Free PMC article. Review.
References
-
- Adair G.S., Block A.V., and Field H.J.. 1925. The hemoglobin system: VI. The oxygen dissociation curve of hemoglobin. J. Biol. Chem. 63:529–545.
-
- Berry J., and Price-Jones M.. 2005. Measurement of radioligand binding by scintillation proximity assay. Methods Mol. Biol. 306:121–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

