Extra Physiotherapy in Critical Care (EPICC) Trial Protocol: a randomised controlled trial of intensive versus standard physical rehabilitation therapy in the critically ill
- PMID: 26009576
- PMCID: PMC4452749
- DOI: 10.1136/bmjopen-2015-008035
Extra Physiotherapy in Critical Care (EPICC) Trial Protocol: a randomised controlled trial of intensive versus standard physical rehabilitation therapy in the critically ill
Abstract
Introduction: Patients discharged from Critical Care suffer from excessive longer term morbidity and mortality. Physical and mental health measures of quality of life show a marked and immediate fall after admission to Critical Care with some recovery over time. However, physical function is still significantly reduced at 6 months. The National Institute for Health and Care Excellence clinical guideline on rehabilitation after critical illness, identified the need for high-quality randomised controlled trials to determine the most effective rehabilitation strategy for critically ill patients at risk of critical illness-associated physical morbidity. In response to this, we will conduct a randomised controlled trial, comparing physiotherapy aimed at early and intensive patient mobilisation with routine care. We hypothesise that this intervention will improve physical outcomes and the mental health and functional well-being of survivors of critical illness.
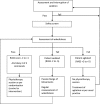
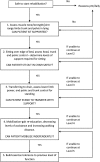
Methods and analysis: 308 adult patients who have received more than 48 h of non-invasive or invasive ventilation in Critical Care will be recruited to a patient-randomised, parallel group, controlled trial, comparing two intensities of physiotherapy. Participants will be randomised to receive either standard or intensive physiotherapy for the duration of their Critical Care admission. Outcomes will be recorded on Critical Care discharge, at 3 and 6 months following initial recruitment to the study. The primary outcome measure is physical health at 6 months, as measured by the SF-36 Physical Component Summary. Secondary outcomes include assessment of mental health, activities of daily living, delirium and ventilator-free days. We will also include a health economic analysis.
Ethics and dissemination: The trial has ethical approval from Newcastle and North Tyneside 2 Research Ethics Committee (11/NE/0206). There is a Trial Oversight Committee including an independent chair. The results of the study will be submitted for publication in peer-reviewed journals and presented at national and international scientific meetings.
Trial registration number: ISRCTN20436833.
Keywords: INTENSIVE & CRITICAL CARE; Physiotherapy.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- NICE. Clinical guideline 83: rehabilitation after critical illness. London: Centre for Clinical Practice at NICE, 2009.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical