Somatodendritic dopamine release: recent mechanistic insights
- PMID: 26009764
- PMCID: PMC4455754
- DOI: 10.1098/rstb.2014.0185
Somatodendritic dopamine release: recent mechanistic insights
Abstract
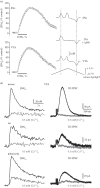
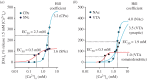
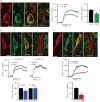
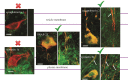
Dopamine (DA) is a key transmitter in motor, reward and cogitative pathways, with DA dysfunction implicated in disorders including Parkinson's disease and addiction. Located in midbrain, DA neurons of the substantia nigra pars compacta project via the medial forebrain bundle to the dorsal striatum (caudate putamen), and DA neurons in the adjacent ventral tegmental area project to the ventral striatum (nucleus accumbens) and prefrontal cortex. In addition to classical vesicular release from axons, midbrain DA neurons exhibit DA release from their cell bodies and dendrites. Somatodendritic DA release leads to activation of D2 DA autoreceptors on DA neurons that inhibit their firing via G-protein-coupled inwardly rectifying K(+) channels. This helps determine patterns of DA signalling at distant axonal release sites. Somatodendritically released DA also acts via volume transmission to extrasynaptic receptors that modulate local transmitter release and neuronal activity in the midbrain. Thus, somatodendritic release is a pivotal intrinsic feature of DA neurons that must be well defined in order to fully understand the physiology and pathophysiology of DA pathways. Here, we review recent mechanistic aspects of somatodendritic DA release, with particular emphasis on the Ca(2+) dependence of release and the potential role of exocytotic proteins.
Keywords: exocytosis; midbrain slices; voltammetry; volume transmission.
© 2015 The Author(s) Published by the Royal Society. All rights reserved.
Figures


Similar articles
-
Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro.J Neurophysiol. 1997 Feb;77(2):853-62. doi: 10.1152/jn.1997.77.2.853. J Neurophysiol. 1997. PMID: 9065854
-
Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum.J Neurosci. 1997 Aug 1;17(15):5738-46. doi: 10.1523/JNEUROSCI.17-15-05738.1997. J Neurosci. 1997. PMID: 9221772 Free PMC article.
-
Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum.J Neurosci. 2001 Oct 1;21(19):7841-7. doi: 10.1523/JNEUROSCI.21-19-07841.2001. J Neurosci. 2001. PMID: 11567075 Free PMC article.
-
Functional interactions between somatodendritic dopamine release, glutamate receptors and brain-derived neurotrophic factor expression in mesencephalic structures of the brain.Brain Res Brain Res Rev. 2004 Dec;47(1-3):126-44. doi: 10.1016/j.brainresrev.2004.05.002. Brain Res Brain Res Rev. 2004. PMID: 15572168 Review.
-
Dopamine release in the basal ganglia.Neuroscience. 2011 Dec 15;198:112-37. doi: 10.1016/j.neuroscience.2011.08.066. Epub 2011 Sep 14. Neuroscience. 2011. PMID: 21939738 Free PMC article. Review.
Cited by
-
Cannabinoid Receptor 1 Is Required for Neurodevelopment of Striosome-Dendron Bouquets.eNeuro. 2022 Apr 11;9(2):ENEURO.0318-21.2022. doi: 10.1523/ENEURO.0318-21.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35361667 Free PMC article.
-
Modulation of dopamine tone induces frequency shifts in cortico-basal ganglia beta oscillations.Nat Commun. 2021 Dec 2;12(1):7026. doi: 10.1038/s41467-021-27375-5. Nat Commun. 2021. PMID: 34857767 Free PMC article.
-
Dopamine Release in the Midbrain Promotes Anxiety.Biol Psychiatry. 2020 Dec 1;88(11):815-817. doi: 10.1016/j.biopsych.2020.08.016. Biol Psychiatry. 2020. PMID: 33153526 Free PMC article. No abstract available.
-
Dopamine neuron-derived IGF-1 controls dopamine neuron firing, skill learning, and exploration.Proc Natl Acad Sci U S A. 2019 Feb 26;116(9):3817-3826. doi: 10.1073/pnas.1806820116. Epub 2019 Feb 11. Proc Natl Acad Sci U S A. 2019. PMID: 30808767 Free PMC article.
-
Impaired dopamine release in Parkinson's disease.Brain. 2023 Aug 1;146(8):3117-3132. doi: 10.1093/brain/awad064. Brain. 2023. PMID: 36864664 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

