Muscarinic inhibition of nicotinic transmission in rat sympathetic neurons and adrenal chromaffin cells
- PMID: 26009767
- PMCID: PMC4455757
- DOI: 10.1098/rstb.2014.0188
Muscarinic inhibition of nicotinic transmission in rat sympathetic neurons and adrenal chromaffin cells
Abstract
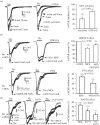
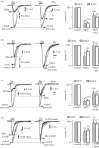
Little is known about the interactions between nicotinic and muscarinic acetylcholine receptors (nAChRs and mAChRs). Here we report that methacholine (MCh), a selective agonist of mAChRs, inhibited up to 80% of nicotine-induced nAChR currents in sympathetic superior cervical ganglion neurons and adrenal chromaffin cells. The muscarine-induced inhibition (MiI) substantially reduced ACh-induced membrane currents through nAChRs and quantal neurotransmitter release. The MiI was time- and temperature-dependent. The slow recovery of nAChR current after washout of MCh, as well as the high value of Q10 (3.2), suggested, instead of a direct open-channel blockade, an intracellular metabotropic process. The effects of GTP-γ-S, GDP-β-S and pertussis toxin suggested that MiI was mediated by G-protein signalling. Inhibitors of protein kinase C (bisindolymaleimide-Bis), protein kinase A (H89) and PIP2 depletion attenuated the MiI, indicating that a second messenger pathway is involved in this process. Taken together, these data suggest that mAChRs negatively modulated nAChRs via a G-protein-mediated second messenger pathway. The time dependence suggests that MiI may provide a novel mechanism for post-synaptic adaptation in all cells/neurons and synapses expressing both types of AChRs.
Keywords: G-proteins; mAchRs; methacholine; nAchRs; nicotine.
© 2015 The Author(s) Published by the Royal Society. All rights reserved.
Figures




Similar articles
-
Desensitization of alpha7 nicotinic receptors potentiated the inhibitory effect on M-current induced by stimulation of muscarinic receptors in rat superior cervical ganglion neurons.J Neural Transm (Vienna). 2005 Sep;112(9):1133-48. doi: 10.1007/s00702-004-0260-6. Epub 2004 Dec 29. J Neural Transm (Vienna). 2005. PMID: 15622441
-
Suppression of the nicotinic acetylcholine response in rat superior cervical ganglionic neurons by steroids.J Neurochem. 1999 Feb;72(2):808-14. doi: 10.1046/j.1471-4159.1999.0720808.x. J Neurochem. 1999. PMID: 9930757
-
Modulation of Ca(2+)-channel currents by protein kinase C in adult rat sympathetic neurons.J Neurophysiol. 1994 Oct;72(4):1549-60. doi: 10.1152/jn.1994.72.4.1549. J Neurophysiol. 1994. PMID: 7823085
-
Modulation of Ca2+ currents by various G protein-coupled receptors in sympathetic neurons of male rat pelvic ganglia.J Neurophysiol. 1997 Aug;78(2):780-9. doi: 10.1152/jn.1997.78.2.780. J Neurophysiol. 1997. PMID: 9307112
-
Muscarinic receptors in adrenal chromaffin cells: physiological role and regulation of ion channels.Pflugers Arch. 2018 Jan;470(1):29-38. doi: 10.1007/s00424-017-2047-2. Epub 2017 Jul 31. Pflugers Arch. 2018. PMID: 28762161 Review.
Cited by
-
Release of chemical transmitters from cell bodies and dendrites of nerve cells.Philos Trans R Soc Lond B Biol Sci. 2015 Jul 5;370(1672):20140181. doi: 10.1098/rstb.2014.0181. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26009760 Free PMC article.
-
Monitoring the Secretory Behavior of the Rat Adrenal Medulla by High-Performance Liquid Chromatography-Based Catecholamine Assay from Slice Supernatants.Front Endocrinol (Lausanne). 2017 Sep 25;8:248. doi: 10.3389/fendo.2017.00248. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28993760 Free PMC article.
References
-
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. 1989. Distribution of alpha2, alpha3, alpha4, and beta2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J. Comp. Neurol. 284, 314–335. (10.1002/cne.902840212) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

