Injection therapies for Achilles tendinopathy
- PMID: 26009861
- PMCID: PMC10804370
- DOI: 10.1002/14651858.CD010960.pub2
Injection therapies for Achilles tendinopathy
Abstract
Background: Achilles tendinopathy is a common condition, often with significant functional consequences. As a wide range of injection treatments are available, a review of randomised trials evaluating injection therapies to help inform treatment decisions is warranted.
Objectives: To assess the effects (benefits and harms) of injection therapies for people with Achilles tendinopathy.
Search methods: We searched the following databases up to 20 April 2015: the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, AMED, CINAHL and SPORTDiscus. We also searched trial registers (29 May 2014) and reference lists of articles to identify additional studies.
Selection criteria: We included randomised and quasi-randomised controlled trials evaluating injection therapies in adults with an investigator-reported diagnosis of Achilles tendinopathy. We accepted comparison arms of placebo (sham) or no injection control, or other active treatment (such as physiotherapy, pharmaceuticals or surgery). Our primary outcomes were function, using measures such as the VISA-A (Victorian Institute of Sport Assessment-Achilles questionnaire), and adverse events.
Data collection and analysis: Two review authors independently extracted data from the included studies. We assessed treatment effects using mean differences (MDs) and 95% confidence intervals (CIs) for continuous variables and risk ratios (RRs) and 95% CIs for dichotomous variables. For follow-up data, we defined short-term as up to six weeks, medium-term as up to three months and longer-term as data beyond three months. We performed meta-analysis where appropriate.
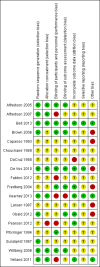
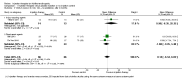
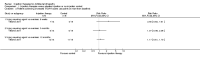
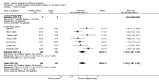
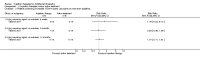
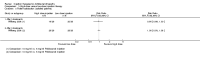
Main results: We included 18 studies (732 participants). Seven trials exclusively studied athletic populations. The mean ages of the participants in the individual trials ranged from 20 years to 50 years. Fifteen trials compared an injection therapy with a placebo injection or no injection control, four trials compared an injection therapy with active treatment, and one compared two different concentrations of the same injection. Thus no trials compared different injection therapies. Two studies had three trial arms and we included them twice in two different categories. Within these categories, we further subdivided injection therapies by mode of action (injury-causing versus direct repair agents).The risk of bias was unclear (due to poor reporting) or high in six trials published between 1987 and 1994. Improved methodology and reporting for the subsequent trials published between 2004 and 2013 meant that these were at less risk of bias.Given the very low quality evidence available from each of four small trials comparing different combinations of injection therapy versus active treatment and the single trial comparing two doses of one injection therapy, only the results of the first comparison (injection therapy versus control) are presented.There is low quality evidence of a lack of significant or clinically important differences in VISA-A scores (0 to 100: best function) between injection therapy and control groups at six weeks (MD 0.79, 95% CI -4.56 to 6.14; 200 participants, five trials), three months (MD -0.94, 95% CI -6.34 to 4.46; 189 participants, five trials) or between six and 12 months (MD 0.14, 95% CI -6.54 to 6.82; 132 participants, three trials). Very low quality evidence from 13 trials showed little difference between the two groups in adverse events (14/243 versus 12/206; RR 0.97, 95% CI 0.50 to 1.89), most of which were minor and short-lasting. The only major adverse event in the injection therapy group was an Achilles tendon rupture, which happened in a trial testing corticosteroid injections. There was very low quality evidence in favour of the injection therapy group in short-term (under three months) pain (219 participants, seven trials) and in the return to sports (335 participants, seven trials). There was very low quality evidence indicating little difference between groups in patient satisfaction with treatment (152 participants, four trials). There was insufficient evidence to conclude on subgroup differences based on mode of action given that only two trials tested injury-causing agents and the clear heterogeneity of the other 13 trials, which tested seven different therapies that act directly on the repair pathway.
Authors' conclusions: There is insufficient evidence from randomised controlled trials to draw conclusions on the use, or to support the routine use, of injection therapies for treating Achilles tendinopathy. This review has highlighted a need for definitive research in the area of injection therapies for Achilles tendinopathy, including in older non-athletic populations. This review has shown that there is a consensus in the literature that placebo-controlled trials are considered the most appropriate trial design.
Conflict of interest statement
Rebecca S Kearney, Nick Parsons, David Metcalfe and Matthew L Costa: the authors' institution, University of Warwick, has received research grants and PRP (platelet‐rich plasma) materials at cost price for studies related to the treatment of Achilles tendinopathy and rupture, including injection studies.
Rebecca S Kearney, Nick Parsons and Matthew L Costa were authors on one of the included study (Kearney 2013). Risk of bias for this trial was independently assessed by David Metcalfe, who had no involvement in this earlier study.
Figures












Update of
- doi: 10.1002/14651858.CD010960
References
References to studies included in this review
Alfredson 2005 {published data only}
-
- Alfredson H, Ohberg L. Sclerosing injections to areas of neo‐vascularisation reduce pain in chronic Achilles tendinopathy: a double blind randomised controlled trial. Knee Surgery, Sports, Traumatology, Arthroscopy 2005;13(4):338‐44. - PubMed
Alfredson 2007 {published data only}
-
- Alfredson H, Ohberg L, Zeisig E, Lorentzon R. Treatment of midportion Achilles tendinosis: similar clinical results with US and CD guided surgery outside the tendon and sclerosing polidocanol injections. Knee, Surgery, Sports, Traumatology, Arthroscopy 2007;15(12):1504‐9. - PubMed
Bell 2013 {published data only}
Brown 2006 {published data only}
Capasso 1993 {published data only}
-
- Capasso G, Maffulli N, Testa V, Sgambato. Preliminary results with peritendinous protease inhibitor injections in the management of Achilles tendinitis. Journal of Sports Traumatology and Related Research 1993;15(1):37–43.
Chouchane 1989 {published data only}
-
- Chouchane A, Commandree F, Fournier S, Plas JN, Viani JL, Zakarian H. Percutaneous treatment of tendinopathy with dexamethasone‐salicyles. Cinesiologie 1989;28:366‐71.
DaCruz 1988 {published data only}
De Vos 2010 {published data only}
-
- Jonge S, Vos RJ, Weir A, Schie HT, Bierma‐Zeinstra S, Verhaar J, et al. One‐year follow‐up of platelet‐rich plasma treatment in chronic Achilles tendinopathy: a double‐blind randomized placebo‐controlled trial. American Journal of Sports Medicine 2011;39(8):1623‐9. - PubMed
-
- Vos R, Weir A, Bierma‐Zeinstra S, Verhaar J, Tol J. Platelet‐rich plasma injection for chronic Achilles tendinopathy: a randomised controlled trial. JAMA 2010;303(2):144‐9. - PubMed
-
- Vos R, Weir A, Verhaar J, Weinans H, Schie H. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. British Journal of Sports Medicine 2011;45(5):387‐92. - PubMed
Fabbro 2012 {published data only}
-
- Fabbro E, Sconfienza LM, Ferrero G, Orlandi D, Lacelli F, Serafini G, et al. One year outcome of ultrasound (US)‐guided percutaneous treatment of Achilles tendinopathy: results of a randomized controlled trial. European Congress of Radiology; 2012 Mar 1‐5; Vienna, Austria. European Society of Radiology Electronic Presentation Online System, 2012:Poster C‐1850. [DOI: 10.1594/ecr2012/C-1850] - DOI
Fredberg 2004 {published data only}
-
- Fredberg U, Bolvig L, Pfeiffer‐Jensen M, Clemmensen D, Jakobsen BW, Stengaard‐Pedersen K. Ultrasonography as a tool for diagnosis, guidance of local steroid injection and, together with pressure algometry, monitoring of the treatment of athletes with chronic jumper's knee and Achilles tendinitis: a randomized, double‐blind, placebo‐controlled study. Scandinavian Journal of Rheumatology 2004;33(2):94‐101. [DOI: 10.1080/03009740310004126] - DOI - PubMed
Kearney 2013 {published data only}
-
- Costa M. Achilles tendinopathy management: platelet‐rich plasma versus eccentric loading programme. http://www.isrctn.com/ISRCTN95369715 (accessed 17 April 2015).
Larsen 1987 {published data only}
-
- Larsen AI, Egfjord M, Jelsdorff HM. Low‐dose heparin in the treatment of calcaneal peritendinitis. Scandinavian Journal of Rheumatology 1987;16(1):47‐51. - PubMed
Obaid 2012 {published data only}
Pearson 2012 {published data only}
-
- Pearson J, Rolands D, Highet R. Autologous blood injection to treat Achilles tendinopathy? A randomized controlled trial. Journal of Sport Rehabilitation 2012;21(3):218‐24. - PubMed
Pforringer 1994 {published data only}
-
- Pforringer W, Pfister A, Kuntz G. The treatment of Achilles paratendinitis: results of a double‐blind, placebo‐controlled study with a deproteinized hemodialysate. Clinical Journal of Sports Medicine 1994;4(2):92‐9.
Sundqvist 1987 {published data only}
-
- Sundqvist H, Forsskahl B, Kvist M. A promising therapy for Achilles peritendinitis: double‐blind comparison of glycosaminoglycan polysulfate and high dose indomethacin. International Journal of Sports Medicine 1987;8(4):298‐303. - PubMed
Willberg 2008 {published data only}
-
- Willberg L, Sunding K, Ohberg L, Forssblad M, Fahlstrom M, Alfredson H. Sclerosis injections to treat midportion Achilles tendinosis: a randomised controlled study evaluating two different concentrations of Polidocanol. Knee Surgery, Sports Traumatology, Arthroscopy 2008;16(9):859‐64. [DOI: 10.1007/s00167-008-0579-x] - DOI - PubMed
Yelland 2011 {published data only}
References to studies excluded from this review
Ferrero 2012 {published data only}
References to studies awaiting assessment
EUCTR2010‐020513‐87 {published data only}
-
- FIDIA. A randomized, placebo‐controlled, double‐blind study on the intensity and duration of efficacy of sodium hyaluronate therapy (500‐730 KDa) (HYALGAN) in the conservative treatment of Achilles tendinopathy. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2010‐020513‐87/IT (accessed 17 November 2014).
Petrella 2013 {published data only}
-
- Petrella R, Petrella M, Decaria J. Hyaluronan alone, combined with botulinus toxin or placebo injection therapy for treatment of chronic noninsertional achilles tendinopathy. Annals of the Rheumatic Diseases 2013;72(Suppl 3):A354.
References to ongoing studies
ISRCTN85334402 {published data only}
-
- Petrou I. A trial evaluating the efficacy of cell therapy based on autologous platelet‐rich plasma (PRP) for the treatment of Achilles and Patellar tendinopathies. http://www.controlled‐trials.com/isrctn/pf/85334402 (accessed 17 November 2014).
NCT01343836 {published data only}
-
- Jonge S. Autologous tenocyte implantation in patients with chronic achilles tendinopathy (ATI). http://clinicaltrials.gov/show/NCT01343836 (accessed 17 November 2014).
NCT01583504 {published data only}
-
- Smith M. High volume saline injections for Achilles tendinopathy. http://clinicaltrials.gov/show/NCT01583504 (accessed 17 November 2014).
NCT01954108 {published data only}
-
- Vroey T, Lynen N. Hyaluronan in the treatment of painful Achilles tendinopathy. http://clinicaltrials.gov/show/NCT01954108 (accessed 17 November 2014).
Additional references
Andres 2008
Coombes 2010
-
- Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet 2010;376(9754):1751‐67. - PubMed
De Jonge 2011
-
- Jonge S, Berg C, Vos R, Heide H, Weir A, Verhaar J, et al. Incidence of midportion Achilles tendinopathy in the general population. British Journal of Sports Medicine 2011;45(13):1026‐8. - PubMed
DTB 2012
-
- Anonymous. Management of chronic Achilles tendinopathy. Drugs and Therapy Bulletin 2012;50(8):93‐6. - PubMed
Evans 2000
-
- Evans NA, Stanish WD. The basic science of tendon injuries. Current Orthopaedics 2000;14(6):403‐12.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kaux 2011
Kearney 2010
-
- Kearney RS, Costa ML. Insertional Achilles tendinopathy management: a systematic review. Foot and Ankle International 2010;31(8):689‐94. - PubMed
Kraemer 2012
-
- Kraemer R, Wuerfel W, Lorenzen J, Busche M, Vogt PM, Knobloch K. Analysis of hereditary and medical risk factors in Achilles tendinopathy and Achilles tendon ruptures: a matched pair analysis. Archives of Orthopaedic and Trauma Surgery 2012;132:847‐53. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Maffulli 2010
-
- Maffulli N, Longo UG, Denaro V. Novel approaches for the management of tendinopathy. Journal of Bone and Joint Surgery ‐ American Volume 2010;92(15):2604‐13. - PubMed
Narici 2008
-
- Narici MV, Maffulli N, Maganaris CN. Ageing of human muscles and tendons. Disability and Rehabilitation 2008;30:1548‐54. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2008
-
- Riley G. Tendinopathy ‐ from basic science to treatment. Nature Clinical Practice Rheumatology 2008;4(2):82‐9. - PubMed
Robinson 2001
Sussmilch‐Leitch 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

