A less-biased analysis of metalloproteins reveals novel zinc coordination geometries
- PMID: 26009987
- PMCID: PMC4539273
- DOI: 10.1002/prot.24834
A less-biased analysis of metalloproteins reveals novel zinc coordination geometries
Abstract
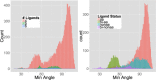
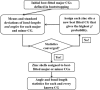
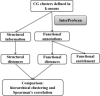
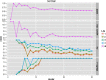
Zinc metalloproteins are involved in many biological processes and play crucial biochemical roles across all domains of life. Local structure around the zinc ion, especially the coordination geometry (CG), is dictated by the protein sequence and is often directly related to the function of the protein. Current methodologies in characterizing zinc metalloproteins' CG consider only previously reported CG models based mainly on nonbiological chemical context. Exceptions to these canonical CG models are either misclassified or discarded as "outliers." Thus, we developed a less-biased method that directly handles potential exceptions without pre-assuming any CG model. Our study shows that numerous exceptions could actually be further classified and that new CG models are needed to characterize them. Also, these new CG models are cross-validated by strong correlation between independent structural and functional annotation distance metrics, which is partially lost if these new CGs models are ignored. Furthermore, these new CG models exhibit functional propensities distinct from the canonical CG models.
Keywords: 3D structure; bidentation; carboxylate shift; compressed angle; structural bioinformatics; structure-function relationship.
© 2015 Wiley Periodicals, Inc.
Figures






Similar articles
-
Aberrant coordination geometries discovered in the most abundant metalloproteins.Proteins. 2017 May;85(5):885-907. doi: 10.1002/prot.25257. Epub 2017 Mar 7. Proteins. 2017. PMID: 28142195 Free PMC article.
-
A bioinformatics view of zinc enzymes.J Inorg Biochem. 2012 Jun;111:150-6. doi: 10.1016/j.jinorgbio.2011.11.020. Epub 2011 Dec 2. J Inorg Biochem. 2012. PMID: 22209023
-
Minimal Functional Sites in Metalloproteins and Their Usage in Structural Bioinformatics.Int J Mol Sci. 2016 May 4;17(5):671. doi: 10.3390/ijms17050671. Int J Mol Sci. 2016. PMID: 27153067 Free PMC article. Review.
-
An integrative computational framework based on a two-step random forest algorithm improves prediction of zinc-binding sites in proteins.PLoS One. 2012;7(11):e49716. doi: 10.1371/journal.pone.0049716. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23166753 Free PMC article.
-
Zinc as Allosteric Ion Channel Modulator: Ionotropic Receptors as Metalloproteins.Int J Mol Sci. 2016 Jul 2;17(7):1059. doi: 10.3390/ijms17071059. Int J Mol Sci. 2016. PMID: 27384555 Free PMC article. Review.
Cited by
-
A chemical interpretation of protein electron density maps in the worldwide protein data bank.PLoS One. 2020 Aug 12;15(8):e0236894. doi: 10.1371/journal.pone.0236894. eCollection 2020. PLoS One. 2020. PMID: 32785279 Free PMC article.
-
Dissociated Hippocampal Neurons Exhibit Distinct Zn2+ Dynamics in a Stimulation-Method-Dependent Manner.ACS Chem Neurosci. 2020 Feb 19;11(4):508-514. doi: 10.1021/acschemneuro.0c00006. Epub 2020 Feb 6. ACS Chem Neurosci. 2020. PMID: 32013397 Free PMC article.
-
Methanethiol Binding Strengths and Deprotonation Energies in Zn(II)-Imidazole Complexes from M05-2X and MP2 Theories: Coordination Number and Geometry Influences Relevant to Zinc Enzymes.J Phys Chem B. 2015 Sep 17;119(37):12182-92. doi: 10.1021/acs.jpcb.5b07115. Epub 2015 Sep 4. J Phys Chem B. 2015. PMID: 26317178 Free PMC article.
-
The Enigmatic Metallothioneins: A Case of Upward-Looking Research.Int J Mol Sci. 2021 Jun 1;22(11):5984. doi: 10.3390/ijms22115984. Int J Mol Sci. 2021. PMID: 34206018 Free PMC article.
-
Correcting the record of structural publications requires joint effort of the community and journal editors.FEBS J. 2016 Dec;283(24):4452-4457. doi: 10.1111/febs.13765. Epub 2016 Jun 10. FEBS J. 2016. PMID: 27229767 Free PMC article.
References
-
- Keilin D, Mann T. Carbonic anhydrase. Nature 1939;144:442–443.
-
- Elrod‐Erickson M, Benson TE, Pabo CO. High‐resolution structures of variant Zif268‐DNA complexes: implications for understanding zinc finger‐DNA recognition. Structure 1998;6:451–464. - PubMed
-
- Carrigan CN, Poulter CD. Zinc is an essential cofactor for type I isopentenyl diphosphate:dimethylallyl diphosphate isomerase. J Am Chem Soc 2003;125:9008–9009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

