IGF1 Promotes Adipogenesis by a Lineage Bias of Endogenous Adipose Stem/Progenitor Cells
- PMID: 26010009
- PMCID: PMC4509822
- DOI: 10.1002/stem.2052
IGF1 Promotes Adipogenesis by a Lineage Bias of Endogenous Adipose Stem/Progenitor Cells
Abstract
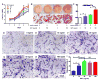
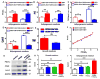
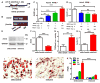
Adipogenesis is essential for soft tissue reconstruction following trauma or tumor resection. We demonstrate that CD31(-)/34(+)/146(-) cells, a subpopulation of the stromal vascular fraction (SVF) of human adipose tissue, were robustly adipogenic. Insulin growth factor-1 (IGF1) promoted a lineage bias towards CD31(-)/34(+)/146(-) cells at the expense of CD31(-)/34(+)/146(+) cells. IGF1 was microencapsulated in poly(lactic-co-glycolic acid) scaffolds and implanted in the inguinal fat pad of C57Bl6 mice. Control-released IGF1 induced remarkable adipogenesis in vivo by recruiting endogenous cells. In comparison with the CD31(-)/34(+)/146(+) cells, CD31(-)/34(+)/146(-) cells had a weaker Wnt/β-catenin signal. IGF1 attenuated Wnt/β-catenin signaling by activating Axin2/PPARγ pathways in SVF cells, suggesting IGF1 promotes CD31(-)/34(+)/146(-) bias through tuning Wnt signal. PPARγ response element (PPRE) in Axin2 promoter was crucial for Axin2 upregulation, suggesting that PPARγ transcriptionally activates Axin2. Together, these findings illustrate an Axin2/PPARγ axis in adipogenesis that is particularly attributable to a lineage bias towards CD31(-)/34(+)/146(-) cells, with implications in adipose regeneration.
Keywords: Adipose; Axin2; CD146; Insulin growth factor-1; Mesenchymal; PPARγ; Stem cells; Stromal vascular fraction.
© 2015 AlphaMed Press.
Figures




References
-
- Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87(3):377–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

