Implementation and Operational Research: A Randomized Noninferiority Trial of AccuCirc Device Versus Mogen Clamp for Early Infant Male Circumcision in Zimbabwe
- PMID: 26010029
- PMCID: PMC4508202
- DOI: 10.1097/QAI.0000000000000694
Implementation and Operational Research: A Randomized Noninferiority Trial of AccuCirc Device Versus Mogen Clamp for Early Infant Male Circumcision in Zimbabwe
Abstract
Background: Early infant male circumcision (EIMC) is a potential key HIV prevention intervention, providing it can be safely and efficiently implemented in sub-Saharan Africa. Here, we present results of a randomized noninferiority trial of EIMC comparing the AccuCirc device with Mogen clamp in Zimbabwe.
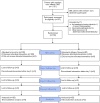
Methods: Between January and June 2013, eligible infants were randomized to EIMC through either AccuCirc or Mogen clamp conducted by a doctor, using a 2:1 allocation ratio. Participants were followed for 14 days post-EIMC. Primary outcomes for the trial were EIMC safety and acceptability.
Results: One hundred fifty male infants were enrolled in the trial and circumcised between 6 and 54 days postpartum (n = 100 AccuCirc; n = 50 Mogen clamp). Twenty-six infants (17%) were born to HIV-infected mothers. We observed 2 moderate adverse events (AEs) [2%, 95% confidence interval (CI): 0.2 to 7.0] in the AccuCirc arm and none (95% CI: 0.0 to 7.1) in the Mogen clamp arm. The cumulative incident risk of AEs was 2.0% higher in the AccuCirc arm compared with the Mogen Clamp arm (95% CI: -0.7 to 4.7). As the 95% CI excludes the predefined noninferiority margin of 6%, the result provides evidence of noninferiority of AccuCirc compared with the Mogen clamp. Nearly all mothers (99.5%) reported great satisfaction with the outcome. All mothers, regardless of arm said they would recommend EIMC to other parents, and would circumcise their next son.
Conclusions: This first randomized trial of AccuCirc versus Mogen clamp for EIMC demonstrated that EIMC using these devices is safe and acceptable to parents. There was no difference in the rate of AEs by device.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- WHO/UNAIDS. New Data on Male Circumcision and HIV Prevention: Policy and Programme Implications. Montreux, Switzerland: WHO/UNAIDS; 2007.
-
- UNICEF. Medical Male Circumcision: Eastern and Southern Africa. 2011. UNICEF/ESARO, Nairobi: Available at: http://www.unicef.org/esaro/5482_7884.html. Accessed June 10, 2011.
-
- Hewett PC, Hallett TB, Mensch BS, et al. Sex with stitches: assessing the resumption of sexual activity during the postcircumcision wound-healing period. AIDS. 2012;26:749–756. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials