MARCKS-dependent mucin clearance and lipid metabolism in ependymal cells are required for maintenance of forebrain homeostasis during aging
- PMID: 26010231
- PMCID: PMC4568964
- DOI: 10.1111/acel.12354
MARCKS-dependent mucin clearance and lipid metabolism in ependymal cells are required for maintenance of forebrain homeostasis during aging
Abstract
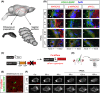
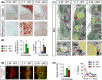
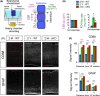
Ependymal cells (ECs) form a barrier responsible for selective movement of fluids and molecules between the cerebrospinal fluid and the central nervous system. Here, we demonstrate that metabolic and barrier functions in ECs decline significantly during aging in mice. The longevity of these functions in part requires the expression of the myristoylated alanine-rich protein kinase C substrate (MARCKS). Both the expression levels and subcellular localization of MARCKS in ECs are markedly transformed during aging. Conditional deletion of MARCKS in ECs induces intracellular accumulation of mucins, elevated oxidative stress, and lipid droplet buildup. These alterations are concomitant with precocious disruption of ependymal barrier function, which results in the elevation of reactive astrocytes, microglia, and macrophages in the interstitial brain tissue of young mutant mice. Interestingly, similar alterations are observed during normal aging in ECs and the forebrain interstitium. Our findings constitute a conceptually new paradigm in the potential role of ECs in the initiation of various conditions and diseases in the aging brain.
Keywords: Clca3; aging; barrier function; cerebral cortex; ependymal cells; lipid droplets; mucin; oxidative stress.
© 2015 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures



Similar articles
-
Phosphorylation-dependent proteome of Marcks in ependyma during aging and behavioral homeostasis in the mouse forebrain.Geroscience. 2022 Aug;44(4):2077-2094. doi: 10.1007/s11357-022-00517-3. Epub 2022 Jan 24. Geroscience. 2022. PMID: 35075585 Free PMC article.
-
Cell type-specific upregulation of myristoylated alanine-rich C kinase substrate and protein kinase C-alpha, -beta I, -beta II, and -delta in microglia following kainic acid-induced seizures.Exp Mol Med. 2006 Jun 30;38(3):310-9. doi: 10.1038/emm.2006.37. Exp Mol Med. 2006. PMID: 16819290
-
CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain.Proc Natl Acad Sci U S A. 2008 Jan 22;105(3):1026-31. doi: 10.1073/pnas.0710000105. Epub 2008 Jan 14. Proc Natl Acad Sci U S A. 2008. PMID: 18195354 Free PMC article.
-
The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread.Toxicol Pathol. 2011 Jan;39(1):186-212. doi: 10.1177/0192623310394214. Epub 2010 Dec 28. Toxicol Pathol. 2011. PMID: 21189316 Review.
-
Ependymal cells and neurodegenerative disease: outcomes of compromised ependymal barrier function.Brain Commun. 2022 Nov 4;4(6):fcac288. doi: 10.1093/braincomms/fcac288. eCollection 2022. Brain Commun. 2022. PMID: 36415662 Free PMC article. Review.
Cited by
-
Glioblastoma disrupts the ependymal wall and extracellular matrix structures of the subventricular zone.Fluids Barriers CNS. 2022 Jul 11;19(1):58. doi: 10.1186/s12987-022-00354-8. Fluids Barriers CNS. 2022. PMID: 35821139 Free PMC article.
-
MARCKS and MARCKS-like proteins in development and regeneration.J Biomed Sci. 2018 May 22;25(1):43. doi: 10.1186/s12929-018-0445-1. J Biomed Sci. 2018. PMID: 29788979 Free PMC article. Review.
-
MARCKS regulates neuritogenesis and interacts with a CDC42 signaling network.Sci Rep. 2018 Sep 5;8(1):13278. doi: 10.1038/s41598-018-31578-0. Sci Rep. 2018. PMID: 30185885 Free PMC article.
-
Ependyma in Neurodegenerative Diseases, Radiation-Induced Brain Injury and as a Therapeutic Target for Neurotrophic Factors.Biomolecules. 2023 Apr 27;13(5):754. doi: 10.3390/biom13050754. Biomolecules. 2023. PMID: 37238624 Free PMC article. Review.
-
Cattle encephalon glycoside and ignotin reduced white matter injury and prevented post-hemorrhagic hydrocephalus in a rat model of intracerebral hemorrhage.Sci Rep. 2016 Oct 26;6:35923. doi: 10.1038/srep35923. Sci Rep. 2016. PMID: 27782218 Free PMC article.
References
-
- Afdhal NH, Cao X, Bansil R, Hong Z, Thompson C, Brown B, Wolf D. Interaction of mucin with cholesterol enriched vesicles: role of mucin structural domains. Biomacromolecules. 2004;5:269–275. - PubMed
-
- Blackshear PJ. The MARCKS family of cellular protein kinase C substrates. J. Biol. Chem. 1993;268:1501–1504. - PubMed
-
- Bouab M, Paliouras GN, Aumont A, Forest-Berard K, Fernandes KJ. Aging of the subventricular zone neural stem cell niche: evidence for quiescence-associated changes between early and mid-adulthood. Neuroscience. 2011;173:135–149. - PubMed
-
- Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol. 2010;119:55–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

