FRAP, FLIM, and FRET: Detection and analysis of cellular dynamics on a molecular scale using fluorescence microscopy
- PMID: 26010322
- PMCID: PMC4515154
- DOI: 10.1002/mrd.22501
FRAP, FLIM, and FRET: Detection and analysis of cellular dynamics on a molecular scale using fluorescence microscopy
Abstract
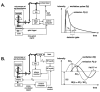
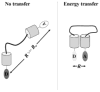
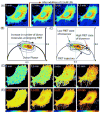
The combination of fluorescent-probe technology plus modern optical microscopes allows investigators to monitor dynamic events in living cells with exquisite temporal and spatial resolution. Fluorescence recovery after photobleaching (FRAP), for example, has long been used to monitor molecular dynamics both within cells and on cellular surfaces. Although bound by the diffraction limit imposed on all optical microscopes, the combination of digital cameras and the application of fluorescence intensity information on large-pixel arrays have allowed such dynamic information to be monitored and quantified. Fluorescence lifetime imaging microscopy (FLIM), on the other hand, utilizes the information from an ensemble of fluorophores to probe changes in the local environment. Using either fluorescence-intensity or lifetime approaches, fluorescence resonance energy transfer (FRET) microscopy provides information about molecular interactions, with Ångstrom resolution. In this review, we summarize the theoretical framework underlying these methods and illustrate their utility in addressing important problems in reproductive and developmental systems.
© 2015 Wiley Periodicals, Inc.
Figures






References
-
- Axelrod D, Wight A, Webb W, Horwitz A. Influence of membrane lipids on acetylcholine receptor and lipid probe diffusion in cultured myotube membrane. Biochemistry. 1978;17:3604–3609. - PubMed
-
- Becker W. Advanced Time-Correlated Single Photon Counting Techniques. Berlin: Springer-Verlag; 2005.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

