Physiologically Based Pharmacokinetic Modeling Is Essential in 90Y-Labeled Anti-CD66 Radioimmunotherapy
- PMID: 26010360
- PMCID: PMC4444288
- DOI: 10.1371/journal.pone.0127934
Physiologically Based Pharmacokinetic Modeling Is Essential in 90Y-Labeled Anti-CD66 Radioimmunotherapy
Abstract
Introduction: Radioimmunotherapy (RIT) with 90Y-labeled anti-CD66 antibody is used to selectively irradiate the red marrow (RM) before blood stem cell transplantation of acute leukemia patients. To calculate the activity to administer, time-integrated activity coefficients are required. These are estimated prior to therapy using gamma camera and serum measurements after injection of 111In labeled anti-CD66 antibody. Equal pre-therapeutic and therapeutic biodistributions are usually assumed to calculate the coefficients. However, additional measurements during therapy had shown that this assumption had to be abandoned. A physiologically based pharmacokinetic (PBPK) model was developed to allow the prediction of therapeutic time-integrated activity coefficients in eight patients.
Aims: The aims of the study were to demonstrate using a larger patient group 1) the need to perform patient-specific dosimetry in 90Y-labeled anti-CD66 RIT, 2) that pre-therapeutic and therapeutic biodistributions differ, and most importantly 3) that this difference in biodistributions can be accurately predicted using a refined model.
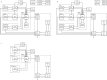
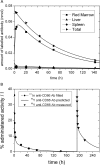
Materials and methods: Two new PBPK models were developed considering fully, half and non-immunoreactive antibodies and constraints for estimating the RM antigen number. Both models were fitted to gamma camera and serum measurements of 27 patients. Akaike weights were used for model averaging. Time-integrated activity coefficients for total body, liver, spleen, RM and serum were calculated. Model-based predictions of the serum biokinetics during therapy were compared to actual measurements.
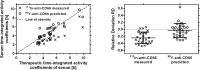
Results: Variability of the RM time-integrated activity coefficients ((37.3 ± 7.5) h) indicates the need for patient-specific dosimetry. The relative differences between pre-therapeutic and therapeutic serum time-activity curves were (-25 ± 16)%. The prediction accuracy of these differences using the refined PBPK models was (-3 ± 20)%.
Conclusion: Individual treatment is needed due to biological differences between patients in RIT with 90Y-labeled anti-CD66 antibody. Differences in pre-therapeutic and therapeutic biokinetics are predominantly caused by different degrees of saturation due to different amounts of administered antibody. These differences could be predicted using the PBPK models.
Conflict of interest statement
Figures
References
-
- Abutalib S.A., Tallman M.S. Monoclonal Antibodies for the Treatment of Acute Myeloid Leukemia. Curr Pharm Biotechnol. 2006;7:343–69. - PubMed
-
- Ringhoffer M, Blumstein N, Neumaier B, Glatting G, von Harsdorf S, Buchmann I, et al. 188Re- or 90Y-labelled anti-CD66 antibody as part of a dose-reduced conditioning regimen for patients with acute leukaemia or myelodysplastic syndrome over the age of 55: results of a phase I-II study. Br J Haematol. 2005;130(4):604–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

