Longitudinal study of informed consent in innovative therapy research: experience and provisional recommendations from a multicenter trial of intracerebral grafting
- PMID: 26010368
- PMCID: PMC4444138
- DOI: 10.1371/journal.pone.0128209
Longitudinal study of informed consent in innovative therapy research: experience and provisional recommendations from a multicenter trial of intracerebral grafting
Abstract
Background: There is an urgent need to assess and improve the consent process in clinical trials of innovative therapies for neurodegenerative disorders.
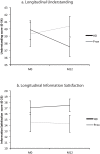
Methods: We performed a longitudinal study of the consent of Huntington's disease patients during the Multicenter Fetal Cell Intracerebral Grafting Trial in Huntington's Disease (MIG-HD) in France and Belgium. Patients and their proxies completed a consent questionnaire at inclusion, before signing the consent form and after one year of follow-up, before randomization and transplantation. The questionnaire explored understanding of the protocol, satisfaction with the information delivered, reasons for participating in the trial and expectations regarding the transplant. Forty-six Huntington's disease patients and 27 proxies completed the questionnaire at inclusion, and 27 Huntington's disease patients and 16 proxies one year later.
Results: The comprehension score was high and similar for Huntington's disease patients and proxies at inclusion (72.6% vs 77.8%; P > 0.1) but only decreased in HD patients after one year. The information satisfaction score was high (73.5% vs 66.5%; P > 0.1) and correlated with understanding in both patients and proxies. The motivation and expectation profiles were similar in patients and proxies and remained unchanged after one year.
Conclusions: Cognitively impaired patients with Huntington's disease were capable of consenting to participation in this trial. This consent procedure has presumably strengthened their understanding and should be proposed before signing the consent form in future gene or cell therapy trials for neurodegenerative disorders. Because of the potential cognitive decline, proxies should be designated as provisional surrogate decision-makers, even in competent patients.
Conflict of interest statement
Figures
Similar articles
-
Positive Attitudes and Therapeutic Misconception Around Hypothetical Clinical Trial Participation in the Huntington's Disease Community.J Huntingtons Dis. 2019;8(4):421-430. doi: 10.3233/JHD-190382. J Huntingtons Dis. 2019. PMID: 31594242 Free PMC article.
-
From open to large-scale randomized cell transplantation trials in Huntington's disease: Lessons from the multicentric intracerebral grafting in Huntington's disease trial (MIG-HD) and previous pilot studies.Prog Brain Res. 2017;230:227-261. doi: 10.1016/bs.pbr.2016.12.011. Epub 2017 Feb 23. Prog Brain Res. 2017. PMID: 28552231
-
Regenerative medicine in Huntington's disease: current status on fetal grafts and prospects for the use of pluripotent stem cell.Rev Neurol (Paris). 2014 Dec;170(12):749-62. doi: 10.1016/j.neurol.2014.10.007. Epub 2014 Nov 4. Rev Neurol (Paris). 2014. PMID: 25459124 Review.
-
How to Capitalize on the Retest Effect in Future Trials on Huntington's Disease.PLoS One. 2015 Dec 29;10(12):e0145842. doi: 10.1371/journal.pone.0145842. eCollection 2015. PLoS One. 2015. PMID: 26714284 Free PMC article. Clinical Trial.
-
The potential of alternate sources of cells for neural grafting in Parkinson's and Huntington's disease.Neurodegener Dis Manag. 2014;4(4):297-307. doi: 10.2217/nmt.14.26. Neurodegener Dis Manag. 2014. PMID: 25313986 Review.
Cited by
-
Positive Attitudes and Therapeutic Misconception Around Hypothetical Clinical Trial Participation in the Huntington's Disease Community.J Huntingtons Dis. 2019;8(4):421-430. doi: 10.3233/JHD-190382. J Huntingtons Dis. 2019. PMID: 31594242 Free PMC article.
-
Protocol for an open label: phase I trial within a cohort of foetal cell transplants in people with Huntington's disease.Brain Commun. 2021 Jan 19;3(1):fcaa230. doi: 10.1093/braincomms/fcaa230. eCollection 2021. Brain Commun. 2021. PMID: 33543141 Free PMC article.
-
Perceptions about Research Participation among Individuals at Risk and Individuals with Premanifest Huntington's Disease: A Survey Conducted by the European Huntington Association.J Pers Med. 2021 Aug 20;11(8):815. doi: 10.3390/jpm11080815. J Pers Med. 2021. PMID: 34442459 Free PMC article.
-
Association Between Motor Symptoms and Brain Metabolism in Early Huntington Disease.JAMA Neurol. 2017 Sep 1;74(9):1088-1096. doi: 10.1001/jamaneurol.2017.1200. JAMA Neurol. 2017. PMID: 28672395 Free PMC article.
-
Future needs for informed consent in stem cell clinical trials in neurodegenerative diseases.Neural Regen Res. 2016 Jan;11(1):83-5. doi: 10.4103/1673-5374.169632. Neural Regen Res. 2016. PMID: 26981090 Free PMC article. No abstract available.
References
-
- Kim SY, Caine ED, Currier GW, Leibovici A, Ryan JM. Assessing the competence of persons with Alzheimer's disease in providing informed consent for participation in research. Am J Psychiatry. 2001. May;158(5):712–7. - PubMed
-
- Sugarman J. Ethics and stem cell therapeutics for cardiovascular disease. Prog Cardiovasc Dis. 2007. Jul-Aug;50(1):1–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

