Potential interactions of calcium-sensitive reagents with zinc ion in different cultured cells
- PMID: 26010609
- PMCID: PMC4444355
- DOI: 10.1371/journal.pone.0127421
Potential interactions of calcium-sensitive reagents with zinc ion in different cultured cells
Abstract
Background: Several chemicals have been widely used to evaluate the involvement of free Ca(2+) in mechanisms underlying a variety of biological responses for decades. Here, we report high reactivity to zinc of well-known Ca(2+)-sensitive reagents in diverse cultured cells.
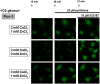
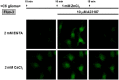
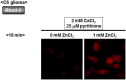
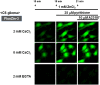
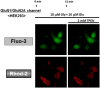
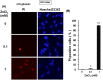
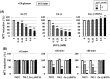
Methodology/principal findings: In rat astrocytic C6 glioma cells loaded with the fluorescent Ca(2+) dye Fluo-3, the addition of ZnCl2 gradually increased the fluorescence intensity in a manner sensitive to the Ca(2+) chelator EGTA irrespective of added CaCl2. The addition of the Ca(2+) ionophore A23187 drastically increased Fluo-3 fluorescence in the absence of ZnCl2, while the addition of the Zn(2+) ionophore pyrithione rapidly and additionally increased the fluorescence in the presence of ZnCl2, but not in its absence. In cells loaded with the zinc dye FluoZin-3 along with Fluo-3, a similarly gradual increase was seen in the fluorescence of Fluo-3, but not of FluoZin-3, in the presence of both CaCl2 and ZnCl2. Further addition of pyrithione drastically increased the fluorescence intensity of both dyes, while the addition of the Zn(2+) chelator N,N,N',N'-tetrakis(2-pyridylmethyl)ethane-1,2-diamine (TPEN) rapidly and drastically decreased FluoZin-3 fluorescence. In cells loaded with FluoZin-3 alone, the addition of ZnCl2 induced a gradual increase in the fluorescence in a fashion independent of added CaCl2 but sensitive to EGTA. Significant inhibition was found in the vitality to reduce 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide in a manner sensitive to TPEN, EDTA and BAPTA in C6 glioma cells exposed to ZnCl2, with pyrithione accelerating the inhibition. Similar inhibition occurred in an EGTA-sensitive fashion after brief exposure to ZnCl2 in pluripotent P19 cells, neuronal Neuro2A cells and microglial BV2 cells, which all expressed mRNA for particular zinc transporters.
Conclusions/significance: Taken together, comprehensive analysis is absolutely required for the demonstration of a variety of physiological and pathological responses mediated by Ca(2+) in diverse cells enriched of Zn(2+).
Conflict of interest statement
Figures










Similar articles
-
The thiol-modifying agent N-ethylmaleimide elevates the cytosolic concentration of free Zn(2+) but not of Ca(2+) in murine cortical neurons.Cell Calcium. 2010 Jul;48(1):37-43. doi: 10.1016/j.ceca.2010.06.004. Epub 2010 Jul 25. Cell Calcium. 2010. PMID: 20667413
-
Zn(2+), derived from cell preparation, partly attenuates Ca(2+)-dependent cell death induced by A23187, calcium ionophore, in rat thymocytes.Toxicol In Vitro. 2009 Mar;23(2):338-45. doi: 10.1016/j.tiv.2008.12.006. Epub 2008 Dec 16. Toxicol In Vitro. 2009. PMID: 19124067
-
Glucose deprivation stimulates Cu(2+) toxicity in cultured cerebellar granule neurons and Cu(2+)-dependent zinc release.Toxicol Lett. 2016 May 27;250-251:29-34. doi: 10.1016/j.toxlet.2016.04.002. Epub 2016 Apr 5. Toxicol Lett. 2016. PMID: 27063646
-
Increase in intracellular Zn2+ concentration by thimerosal in rat thymocytes: intracellular Zn2+ release induced by oxidative stress.Toxicol In Vitro. 2009 Sep;23(6):1092-9. doi: 10.1016/j.tiv.2009.05.020. Epub 2009 Jun 2. Toxicol In Vitro. 2009. PMID: 19497362
-
Zinc flexes its muscle: Correcting a novel analysis of calcium for zinc interference uncovers a method to measure zinc.J Gen Physiol. 2016 Jan;147(1):95-102. doi: 10.1085/jgp.201511493. J Gen Physiol. 2016. PMID: 26712852 Free PMC article.
Cited by
-
Evaluating the Utilization of Ethylenediaminetetraacetic Acid as a Treatment Supplement for Gliomas.Cureus. 2022 Nov 17;14(11):e31617. doi: 10.7759/cureus.31617. eCollection 2022 Nov. Cureus. 2022. PMID: 36540522 Free PMC article. Review.
-
The role of Zn2+ in shaping intracellular Ca2+ dynamics in the heart.J Gen Physiol. 2023 Jul 3;155(7):e202213206. doi: 10.1085/jgp.202213206. Epub 2023 Jun 16. J Gen Physiol. 2023. PMID: 37326614 Free PMC article. Review.
-
Agonist-Evoked Increases in Intra-Platelet Zinc Couple to Functional Responses.Thromb Haemost. 2019 Jan;119(1):128-139. doi: 10.1055/s-0038-1676589. Epub 2018 Dec 31. Thromb Haemost. 2019. PMID: 30597507 Free PMC article.
References
-
- Scheetz AJ, Constantine-Paton M (1994) Modulation of NMDA receptor function: implications for vertebrate neural development. FASEB J. 8: 745–752. - PubMed
-
- Collingridge GL, Bliss TV (1995) Memories of NMDA receptors and LTP. Trends Neurosci 18: 54–56. - PubMed
-
- Choi DW (1988) Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci 11: 465–469. - PubMed
-
- Sattler R, Tymianski M (2000) Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med 78: 3–13. - PubMed
-
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL (1986) NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature 321, 519–522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

