Optogenetic excitation of preBötzinger complex neurons potently drives inspiratory activity in vivo
- PMID: 26010654
- PMCID: PMC4560590
- DOI: 10.1113/JP270471
Optogenetic excitation of preBötzinger complex neurons potently drives inspiratory activity in vivo
Abstract
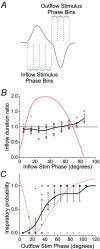
Key points: This study investigates the effects on ventilation of an excitatory stimulus delivered in a spatially and temporally precise manner to the inspiratory oscillator, the preBötzinger complex (preBötC). We used an adeno-associated virus expressing channelrhodopsin driven by the synapsin promoter to target the region of the preBötC. Unilateral optogenetic stimulation of preBötC increased respiratory rate, minute ventilation and increased inspiratory modulated genioglossus muscle activity. Unilateral optogenetic stimulation of preBötC consistently entrained respiratory rate up to 180 breaths min(-1) both in presence of ongoing respiratory activity and in absence of inspiratory activity. Unilateral optogenetic stimulation of preBötC induced a strong phase-independent Type 0 respiratory reset, with a short delay in the response of 100 ms. We identified a refractory period of ∼200 ms where unilateral preBötC optogenetic stimulation is not able to initiate the next respiratory event.
Abstract: Understanding the sites and mechanisms underlying respiratory rhythmogenesis is of fundamental interest in the field of respiratory neurophysiology. Previous studies demonstrated the necessary and sufficient role of preBötzinger complex (preBötC) in generating inspiratory rhythms in vitro and in vivo. However, the influence of timed activation of the preBötC network in vivo is as yet unknown given the experimental approaches previously used. By unilaterally infecting preBötC neurons using an adeno-associated virus expressing channelrhodopsin we photo-activated the network in order to assess how excitation delivered in a spatially and temporally precise manner to the inspiratory oscillator influences ongoing breathing rhythms and related muscular activity in urethane-anaesthetized rats. We hypothesized that if an excitatory drive is necessary for rhythmogenesis and burst initiation, photo-activation of preBötC not only will increase respiratory rate, but also entrain it over a wide range of frequencies with fast onset, and have little effect on ongoing respiratory rhythm if a stimulus is delivered during inspiration. Stimulation of preBötC neurons consistently increased respiratory rate and entrained respiration up to fourfold baseline conditions. Furthermore, brief pulses of photostimulation delivered at random phases between inspiratory events robustly and consistently induced phase-independent (Type 0) respiratory reset and recruited inspiratory muscle activity at very short delays (∼100 ms). A 200 ms refractory period following inspiration was also identified. These data provide strong evidence for a fine control of inspiratory activity in the preBötC and provide further evidence that the preBötC network constitutes the fundamental oscillator of inspiratory rhythms.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures
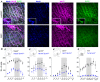

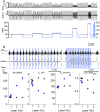
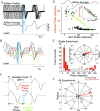
 ) and normalized changes in DIAEMG and GGEMG activity upon CP (circles) and HF photostimulation (squares). *Statistically significant changes (P<0.05) compared to pre-stimulation values.
) and normalized changes in DIAEMG and GGEMG activity upon CP (circles) and HF photostimulation (squares). *Statistically significant changes (P<0.05) compared to pre-stimulation values.


Similar articles
-
Optogenetic perturbation of preBötzinger complex inhibitory neurons modulates respiratory pattern.Nat Neurosci. 2015 Mar;18(3):408-14. doi: 10.1038/nn.3938. Epub 2015 Feb 2. Nat Neurosci. 2015. PMID: 25643296 Free PMC article.
-
Phase- and state-dependent modulation of breathing pattern by preBötzinger complex somatostatin expressing neurons.J Physiol. 2022 Jan;600(1):143-165. doi: 10.1113/JP282002. Epub 2021 Dec 8. J Physiol. 2022. PMID: 34783033 Free PMC article.
-
Dendritic A-Current in Rhythmically Active PreBötzinger Complex Neurons in Organotypic Cultures from Newborn Mice.J Neurosci. 2018 Mar 21;38(12):3039-3049. doi: 10.1523/JNEUROSCI.3342-17.2018. Epub 2018 Feb 19. J Neurosci. 2018. PMID: 29459371 Free PMC article.
-
Pre-Bötzinger complex: Generation and modulation of respiratory rhythm.Neurologia (Engl Ed). 2019 Sep;34(7):461-468. doi: 10.1016/j.nrl.2016.05.011. Epub 2016 Jul 18. Neurologia (Engl Ed). 2019. PMID: 27443242 Review. English, Spanish.
-
Respiratory Rhythm Generation: The Whole Is Greater Than the Sum of the Parts.Adv Exp Med Biol. 2017;1015:147-161. doi: 10.1007/978-3-319-62817-2_9. Adv Exp Med Biol. 2017. PMID: 29080026 Review.
Cited by
-
Activation of the periaqueductal gray controls respiratory output through a distributed brain network.Front Physiol. 2025 Jan 22;16:1516771. doi: 10.3389/fphys.2025.1516771. eCollection 2025. Front Physiol. 2025. PMID: 39911274 Free PMC article.
-
PreBötzinger complex neurons drive respiratory modulation of blood pressure and heart rate.Elife. 2020 Jun 15;9:e57288. doi: 10.7554/eLife.57288. Elife. 2020. PMID: 32538785 Free PMC article.
-
Perturbations of Respiratory Rhythm and Pattern by Disrupting Synaptic Inhibition within Pre-Bötzinger and Bötzinger Complexes.eNeuro. 2016 May 13;3(2):ENEURO.0011-16.2016. doi: 10.1523/ENEURO.0011-16.2016. eCollection 2016 Mar-Apr. eNeuro. 2016. PMID: 27200412 Free PMC article.
-
Vasopressin and Breathing: Review of Evidence for Respiratory Effects of the Antidiuretic Hormone.Front Physiol. 2021 Oct 26;12:744177. doi: 10.3389/fphys.2021.744177. eCollection 2021. Front Physiol. 2021. PMID: 34867449 Free PMC article. Review.
-
Organization of the core respiratory network: Insights from optogenetic and modeling studies.PLoS Comput Biol. 2018 Apr 26;14(4):e1006148. doi: 10.1371/journal.pcbi.1006148. eCollection 2018 Apr. PLoS Comput Biol. 2018. PMID: 29698394 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

