Projections from the subparaventricular zone define four channels of output from the circadian timing system
- PMID: 26010698
- PMCID: PMC4607558
- DOI: 10.1002/cne.23812
Projections from the subparaventricular zone define four channels of output from the circadian timing system
Abstract
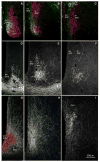
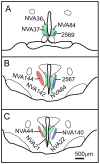
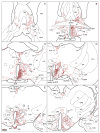
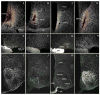
The subparaventricular zone of the hypothalamus (SPZ) is the main efferent target of neural projections from the suprachiasmatic nucleus (SCN) and an important relay for the circadian timing system. Although the SPZ is fairly homogeneous cytoarchitecturally and neurochemically, it has been divided into distinct functional and connectional subdivisions. The dorsal subdivision of the SPZ (dSPZ) plays an important role in relaying signals from the SCN controlling body temperature rhythms, while the ventral subdivision (vSPZ) is critical for rhythms of sleep and locomotor activity (Lu et al. [] J Neurosci 21:4864-4874). On the other hand, the medial part of the SPZ receives input mainly from the dorsomedial SCN, whereas the lateral SPZ receives input from the ventrolateral SCN and the retinohypothalamic tract (Leak and Moore [] J Comp Neurol 433:312-334). We therefore investigated whether there are corresponding differences in efferent outputs from these four quadrants of the SPZ (dorsolateral, ventrolateral, dorsomedial, and ventromedial) by a combination of anterograde and retrograde tracing. We found that, while all four subdivisions of the SPZ share a similar backbone of major projection pathways to the septal region, thalamus, hypothalamus, and brainstem, each segment of the SPZ has a specific set of targets where its projections dominate. Furthermore, we observed intra-SPZ projections of varying densities between the four subdivisions. Taken together, this pattern of organization suggests that the circadian timing system may have several parallel neural outflow pathways that provide a road map for understanding how they subserve different functions.
Keywords: circadian timing; neural projections; subparaventricular zone.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures





References
-
- Belenky MA, Yarom Y, Pickard GE. Heterogeneous expression of gamma-aminobutyric acid and gamma-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. The Journal of comparative neurology. 2008;506(4):708–732. - PubMed
-
- Cambras T, Weller JR, Angles-Pujoras M, Lee ML, Christopher A, Diez-Noguera A, Krueger JM, de la Iglesia HO. Circadian desynchronization of core body temperature and sleep stages in the rat. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(18):7634–7639. - PMC - PubMed
-
- Canteras NS, Ribeiro-Barbosa ER, Goto M, Cipolla-Neto J, Swanson LW. The retinohypothalamic tract: comparison of axonal projection patterns from four major targets. Brain research reviews. 2011;65(2):150–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

